1596
How far Should Coil Coverage be Extended to Reach Optimum Simultaneous Multi-Slice Acceleration in Cardiac MRI?
Anpreet Ghotra1, Sam-Luca JD Hansen1, Robin Etzel1, Mirsad Mahmutovic1, Alina Scholz1, Nicolas Kutscha1, Matthäus Poniatowski1, Markus W May1, Choukri Mekkaoui2, and Boris Keil1
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Harvard Medical School, Massachusetts General Hospital, Department of Radiology, A.A. Martinos Center for Biomedical Imaging, Boston, MA, United States
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Harvard Medical School, Massachusetts General Hospital, Department of Radiology, A.A. Martinos Center for Biomedical Imaging, Boston, MA, United States
Synopsis
Even with commonly used k-space subsampling acceleration methods, cardiac MRI still suffers from relatively slow acquisition speed which imposes limitations in spatial and temporal resolution and volumetric coverage when imaging the moving heart. The recently introduced simultaneous multi-slice (SMS) acceleration technique offers the potential to acquire multiple slices which can substantially increase myocardial coverage without compromising in-plane spatial resolution. However, to disentangle the collapsed slices, array coils must provide enough sensitivity variation along the slice direction. Therefore, in this simulation study, we evaluated the coverage of the cardiac array coil to maximize SMS encoding performance.
Introduction
Accelerated parallel imaging techniques have impacted clinical applications such that every cardiac MRI examination is performed with a surface coil array comprising multiple small receive elements. Where only a small number of receiving channels are available (<16 channels), several cardiac coil studies have shown that the array should be positioned concentrically above and below the heart1,2,3, including a slight left-sided 4. However, recent studies with larger channel counts (>32 channels) suggested that coil elements should radially encompass the upper thorax to obtain optimal sensitivity of the cardiac region. The recently introduced simultaneous multi-slice (SMS) acceleration techniques5,6 seem to be very promising for cardiac MRI7. Unlike in-plane acceleration methods, SMS shares the advantage of 3D volume data sampling and has no additional SNR penalty due to skipped k-space lines, other than the g‐factor reconstruction noise. This imposes new optimization constraints for the array coil design to ensure adequate sensitivity variation along the slice direction and, more importantly, to determine how far the coil elements should be extended for a given target region. Therefore, the primary rationale of this simulation study was to find out the role of the elements distal to the heart in highly SMS accelerated cardiac imaging.Methods
We constraint our simulation to 64 channels and a radially enclosed array coil topology to be placed on the upper torso and centered over the heart. The loading torso phantom including the heart, the coil former, and the overlapped coil elements were modeled in a computer-aided design (CAD) software (Rhino3D, Robert McNeel & Associates, Seattle, USA) (Fig. 1). Given the geometrical constraints and the anatomical coverage for each layout, the elements were sized appropriately to meet the desired number of 64 channels: for each array configuration A, B, C, and D, the loop diameters resulted in 52mm, 75mm, 90mm, and 120mm, respectively.The open-source full-wave simulation package MARIE8,9 was used to compute the sensitivity and acceleration capability of the arrays. Each loop layout and the conductive dielectric-loading model (load of σ=0.49 S/m, εr=66.34) were imported to MARIE. Excitation was emulated using a sinusoidal unit current at the Larmor frequency of 123.25MHz. The complex reception profile of each coil array was taken as the B1- component of the simulated field for 60 transverse planes, which corresponds to a two-chamber slice prescription of the heart. However, simulations did not account for coil tuning or matching conditions and neglected inter-element coupling. We also calculated each array’s mutual noise resistance matrix from the inner product of the spatially varying electric fields of the coils over the sample volume. The relative noise levels were obtained from Q-ratio measurements of coil loops constructed to match given loop sizes. These uncorrelated noise levels were then added to the diagonal terms of the resistance matrix10.
We computed the unaccelerated and accelerated image signal-to-noise ratio (SNR). Noise amplifications (g-factors) were obtained using the formulation for SENSE reconstruction11. This concept was extended to derive g-factors for SMS acquisitions.
Results
Layout B shows the overall best unaccelerated SNR in the target volume (Fig. 2). Although Layout A has larger peak SNR at the anterior heart region, it shows lowest SNR performance at the posterior heart region when compared with all array configurations. Coil arrays C and D provide slightly lower SNR performance than Layout B.In terms of encoding power for the heart region (Fig. 3), Layout D showed favorable g-factors for multiband factors MB=4, MB=6, and MB=8 with and without in-plane acceleration. Layout B slightly outperforms array configurations A and C. When taking into account the baseline SNR and the local noise amplifications, the resulted accelerated SNR is a more meaningful metric for imaging performance. In this context, Layout B shows the highest peak SNR (Fig. 4). However, layout D offers the highest low-point SNR, compared with the other coil arrangements. Imaging the posterior region of the heart is associated with the lowest reception sensitivity of the coils. At this challenging region, Layout D outperforms every other simulated coil configuration (Fig. 5).
Discussion
Although higher channel-count arrays in cardiac imaging are well-understood12,13, there are additional challenges present when an optimal coil encoding structure must be found for SMS acceleration techniques. This renders the array coil design to new optimization constraints. The simulated coil arrays which compactly covered only the heart region (A,B) showed highest unaccelerated myocardial SNR. For the arrays with extended coverage (C,D), many of the additional elements distal to the heart contributed less to the myocardial SNR in unaccelerated imaging. However, SMS g-maps showed a significant role for distal elements in highly accelerated cardiac imaging, resulting in higher SNR especially at the posterior myocardial wall. Simulations suggest the coil coverage should be extended by approximate 8cm above and below the heart for highly accelerated cardiac imaging performance.Conclusion
For highly SMS accelerated cardiac imaging, 64 channels coil arrays with extended coverage can produce substantial more favorable encoding capacities than arrays which compactly encompass the heart region.Acknowledgements
This project was funded by BMBF (German Gov’t funding, ID: IN2016-2-226).References
- Constantinides, Chris D., et al. A phased array coil for human cardiac imaging. Magn Reson Med. 34.1 (1995): 92-98.
- Niendorf T., et al. Towards whole-heart coverage in a single breath hold: coronary artery imaging using a true 32-channel phased-array MRI system; Proceedings of the 12th Annual Meeting of ISMRM; Kyoto, Japan. 2004. p. 703.
- Hardy, Christopher J., et al. 32‐element receiver‐coil array for cardiac imaging. Magn Reson Med. 55.5 (2006): 1142-1149.
- Bottomley, Paul A., et al. What is the optimum phased array coil design for cardiac and torso magnetic resonance?. Magn Reson Med. 37.4 (1997): 591-599.
- Setsompop K., et al. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for Simultaneous Multislice EPI With Reduced g-Factor Penalty. Magn Reson Med. 2012;67:1210-1224.
- Feinberg DA, et al. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS ONE. 2010;5(12).
- Choukri Mekkaoui, et al. Diffusion tractography of the entire left ventricle by using free-breathing accelerated simultaneous multisection imaging. Radiology 282.3 (2017): 850-856.
- Polimeridis A., et al. Stable FFT-JVIE solvers for fast analysis of highly inhomogeneous dielectric objects. J Comput Phys. 2014;269:280–96.
- Villena JF., et al. MARIE – a MATLAB-based open source software for the fast electromagnetic analysis of MRI systems. In proc ISMRM. 2015;23:0709.
- King, Scott B., et al. Eigenmode analysis for understanding phased array coils and their limits. Concepts in Magnetic Resonance Part B: Magnetic Resonance Engineering: An Educational Journal 29.1 (2006): 42-49.
- Pruessmann, Klaas P., et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 42.5 (1999): 952-962.
- Schmitt, Melanie, et al. A 128‐channel receive‐only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn Reson Med. 59.6 (2008): 1431-1439.
- Etzel, R., X. Cao, and C. Mekkaoui. Design optimization and evaluation of a 64‐channel cardiac array coil at 3T. Proc 23rd Annual Meeting ISMRM, Toronto. 2015.
Figures
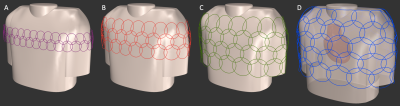
Modelled and simulated circular overlapped coil arrays for highly SMS accelerated cardiac images.
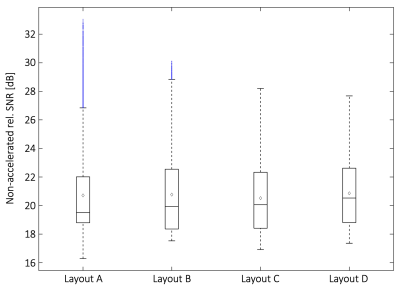
Unaccelerated SNR obtained from the cardiac target region (the blue marked values are considered as outliers in boxplot statistics).
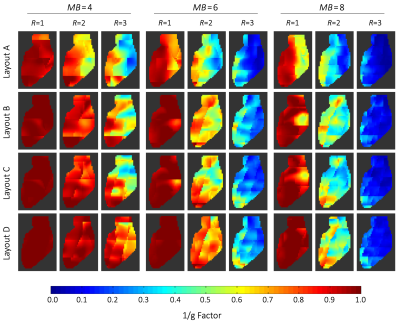
Inverse g-Factor maps of a representative sagittal slice through the heart. g-factors were derived from axial SMS accelerations with multiband factors 4, 6, 8, and additionally combined with in-plane accelerations of R=2 and R=3. The heart region was covered by 60 slices of 2 mm each. 15, 10, and 8 collapsed slices were needed to reconstruct the full heart for MB=4, MB=6, and MB=8, respectively.

Accelerated SNR for different SMS factors MB and in-plane accelerations R obtained from whole heart target region (Note, R=1 correspond to no in-plane acceleration).
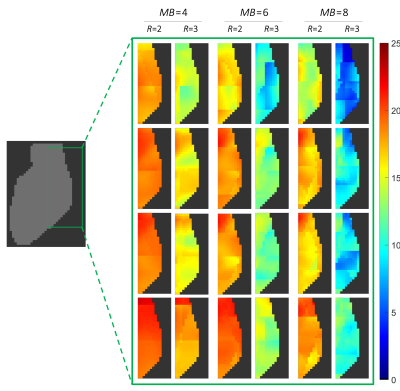
Accelerated SNR of the posterior region of the heart for different SMS factors (MB=4, MB=6 and MB=8) and in-plane accelerations (R=2, R=3).