1593
A Quadrature Birdcage/47Rx Coil Array for Acceleration Images on 3 T MRI1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen Key Laboratory for MRI, Shenzhen, China, 3United Imaging Healthcare, Shanghai, China, 4Department of Biomedical Engineering, State University of New York, Buffalo, NY, United States
Synopsis
We designed and built a quadrature-birdcage/ 47Rx head coil array for accelerated images on 3 T MRI. A 32-channel commercial head coil was used as a comparison. The inverse g-factor images and accelerated anatomical images both show that the 47-channel head coil has better acceleration ability. EPI images and cerebrovascular images further verified that the 47-channel head coil is capable of human’s brain studies.
Introduction
The relatively long scan time of MRI causes several challenges in research and clinical diagnosis of human brain [1]. Radiofrequency (RF) coil arrays were considered as a useful tool for supporting parallel imaging methods to reduce the scan time. RF coils with higher channel numbers have better acceleration capability [2]. Also, the large field-of-view (FOV) offered by the volume coil might bring about the artifacts in certain parallel imaging methods [3]. Therefore, we designed and built a head coil contains a quadrature-birdcage for local transmit and a 47Rx-receive array for high acceleration ability. To investigate the acceleration ability, inverse g-factor maps and anatomical images under acceleration of human brain were obtained.Methods
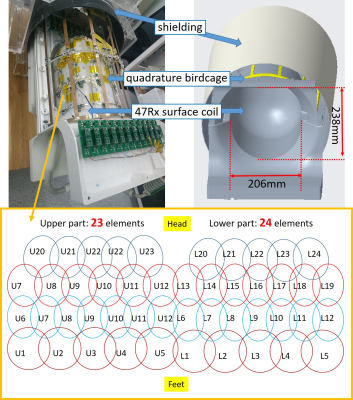
The quadrature-birdcage/47Rx-loops array was arranged on a Polycarbonate-made structure (Shenzhen En Xin Long super engineering plastics Co., LTD), as shown in Fig. 1. The birdcage was made 31 cm in diameter and 39 cm in length to completely cover the human’s head for local transmit. The coil structure was designed with 20.8 cm in inner diameter and 23.8 cm in inner length to close-fit human’s head. 47 loops were selected to reach the maximum coil number available for the MRI system, and was arranged with 23 on the upper part and 24 at the bottom part.All on system studies were performed on a 3 T MRI system (uMR 790, United Imaging Healthcare, Shanghai, China), and has been compared to a 32-channel commercial head coil to quantitatively analyze coil performances. Two volunteers (men, age 40-year-old, weight 70 kg; women, age 25-year-old, weight 55 kg) were scanned during the experiments.
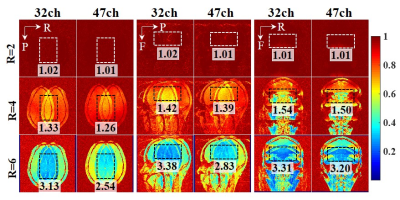
Gradient Echo (GRE) images (field of view (FOV) = 220 mm × 220 mm, acquisition matrix = 272 × 272, slice thickness = 1.8 mm, repetition time (TR) = 300 ms, echo time (TE) = 6.48 ms, bandwidth = 200 Hz/pixel, and flip angle (FA) = 30 degrees) were used to analyze g-factor performances. The FOV was set tightly to avoid underestimation of the g-factor. The inverse g-factor maps were analyzed by using sensitivity encoding (SENSE) reconstructions [4].
Compressed sensing accelerated GRE Time-of-flight (TOF) images (FOV = 220 mm (readout direction) × 180 mm (phase encoding direction), acquisition matrix = 368 × 294, slices per slab = 40, slice thickness = 1 mm, TR/TE = 19 /4ms, bandwidth = 250 Hz/pixel, and FA = 18 degrees) were used to observe the ability for coils to attain cerebrovascular images.
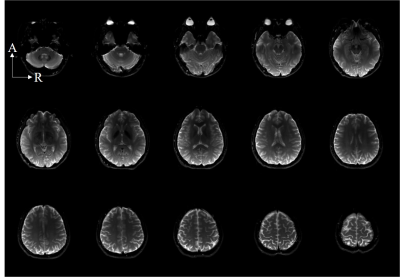
Echoplanar imaging (EPI) sequences (FOV = 230 mm × 220 mm, acquisition matrix = 160 × 160, slice thickness = 5 mm, TR/TE = 2400/81 ms, bandwidth = 1790 Hz/pixel, and FA = 90 degrees, echo trains length = 69) were used to acquire whole images for functional MRI application.
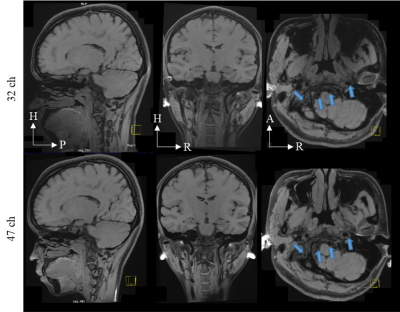
Modulated flip angle technique in refocused imaging with extended echo train (MATRIX) were used to acquire accelerated anatomical images. The sequence parameters were set as follow: slices per slab = 256, slice thickness = 0.6 mm, FOV = 220 mm (readout direction) Х 192 mm (phase encoding direction), TR/TE=850/12.9 ms, Echo train length=46, Bandwidth=600Hz/Pixel.
Results/Discussion
The inverse g-factor maps are shown in Fig. 2. The acceleration factors ranged from 2 to 6. The acceleration directions are selected alien in each orientation, which is: Left-to-Right acceleration direction in transverse orientation, Anterior-to-Posterior acceleration direction in sagittal orientation, and Head-to-Feet acceleration direction in coronal orientation. The ROI was selected as the brain area. The mean g-factor value in the selected ROI is annotated in images. The differences between the two coils were up to 19% in the high acceleration factor.The accelerated anatomical images were shown in Fig. 3. The acceleration direction was on phase encoding and slice phase encoding with an acceleration factor of 7 in total. The 47-channel head coil shows better image quality in the carotid artery area.
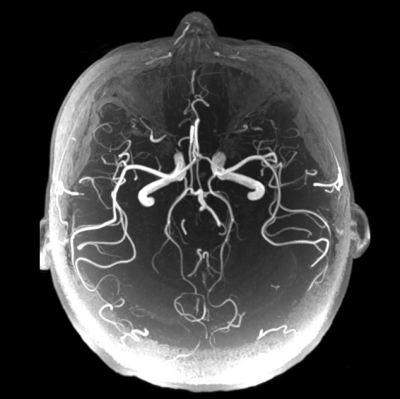
Fig.4 and Fig. 5 shows the ability of the 47-channel head coil to acquire whole-brain EPI images and cerebrovascular images of human brain.
Conclusion
A quadrature birdcage/47Rx head coil array was presented and tested on a 3 T MRI system for human brain studies. Compared with the commercial 32-channel head coil, which was often used for human brain imaging experiments, the 47-channel head coil improved the parallel imaging performance in all directions with up to an acceleration factor of 6. In in-vivo images, the anatomical images acquired by the 47-channel head coil show better image quality under high acceleration factor. The results show that the 47-channel head coil might be able to apply on more accelerated imaging acquisitions in the future.Acknowledgements
This work was supported in part by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB25000000), Guangdong Province grants 2018B030333001, NSFC under Grant No. 81627901. Youth Innovation Promotion Association of CAS No. 2017415, and National Natural science foundation of China (Grant No.: 81671789)References
[1] Polak, Daniel, et al. "Wave‐CAIPI for highly accelerated MP‐RAGE imaging." Magnetic resonance in medicine 79.1 (2018): 401-406.
[2] Gao, Yang, Weidao Chen, and Xiaotong Zhang. "Investigating the influence of spatial constraints on ultimate receive coil performance for monkey brain MRI at 7 T." IEEE transactions on medical imaging 37.7 (2018): 1723-1732.
[3] Polak, Daniel, et al. "Highly‐accelerated volumetric brain examination using optimized wave‐CAIPI encoding." Journal of Magnetic Resonance Imaging 50.3 (2019): 961-974.
[4] K. P. Pruessmann, M. Weiger, M. B. Scheidegger, and P. Boesiger, "SENSE: sensitivity encoding for fast MRI," Magn Reson Med, vol. 42, no.5, pp. 952-962, Oct. 1999.
Figures