1556
Golden-angle radial MR fingerprinting for high-resolution quantitative prostate MRI1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Philips Healthcare, Hamburg, Germany
Synopsis
We demonstrated the feasibility of golden-angle radial MR fingerprinting for high-resolution quantitative multi-parametic prostate MRI. The variation in T1 and T2 values for different scan times within the normal prostate and the required number of spokes per temporal time frame required for robust quantitation parameter mapping were examined. A decrease in average T1 and T2 values was observed as scan time increased. Radial spokes of 2 or more per temporal frame achieved parameter maps that are in good agreement with reported normal prostate transition zone values.
Introduction
Magnetic resonance fingerprinting (MRF) enables for robust simultaneous quantitative multiparametric mapping in a single acquisition (1,2). Feasibility of combining T1 and T2 maps with apparent diffusion coefficient (ADC) maps for improving diagnosis of prostate cancer have been demonstrated (3,4). MRF is usually performed with spiral acquisitions, which may be sensitive to B0 inhomogeneities in the prostate region due to long readouts. Although most MRF studies use a spiral sampling of k-space (1), radial sampling is attractive due to its inherent robustness to motion and short echo times which mitigate B0 inhomogeneity effects. One of the drawbacks of radial imaging is to match the imaging speed of spiral imaging for MRF. This work explores the utilization of highly-accelerated golden-angle radial acquisition for MRF in the prostate and studies the required number of spokes per temporal frame to achieve robust and high resolution parametric mapping.Methods
A 2D steady-state-free-precession (SSFP) MRF sequence with inversion preparation was used with golden angle radial trajectory readout (5). All scans were performed on a 3T Philips Elition (Philips, Best, The Netherlands) running software version 5.6 and equipped with a flat tabletop for MRI simulation. MR fingerprinting imaging parameters are as follows: FOV = 250 x 250 mm2, image matrix = 224 x 224, slice thickness = 5 mm, echo time (TE) = 4 ms, repetition time (TR) = 8 ms, variable flip angle range = 0-60 degrees. The utilized acquisition schedule contains 500 time points. The radial MRF sequence was tested with the NIST quantitative imaging phantom (6) and the measured T1 and T2 values validated against the ground truth values. A healthy 23-year-old subject was recruited and gave informed consent in accordance with institutional IRB. Acquisitions with 1, 2, 3, and 6 radial spokes per time point were performed. T1, and T2 relaxation parameter maps were calculated by dot product matching to the simulated MRF dictionary. The normal prostate was delineated, and the statistics and distribution of the quantitative mapping values were analyzed.Results
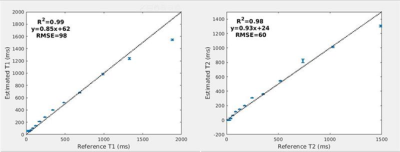
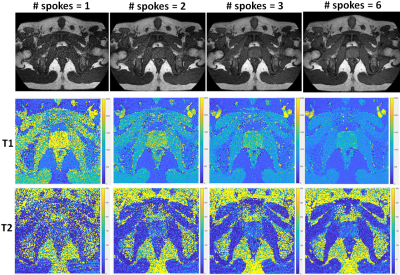
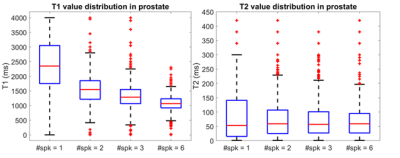
Figure 1 illustrates the NIST phantom T1 T2 mapping validation of the utilized acquisition, reconstruction, and dictionary matching pipeline. The NIST phantom validation demonstrated good agreement between expected and acquired T1 and T2 values. For the in-vivo acquisition, all examined acceleration levels (number of radial spokes per time point) yielded images with high geometric integrity. The resultant images and quantitative maps are shown in Figure 2. The mean and standard deviation of T1 and T2 values within the prostate region, and the corresponding acquisition times are shown in Table 1. Figure 3 demonstrates the change in the distribution of the parameter maps in relation to acceleration level in boxplots. As expected, a decrease in noise in the reconstructed images was observed as the number of spokes increased. Average T1 values noticeably decreased as the number of spokes increased. Average T2 values do not exhibit as substantial of change but the distribution and spread of the values reduce significantly as number of spokes increase. From the visualization of the quantitative maps as shown in Figure 2, the T1 and T2 mapping breaks down for the acquisitions with radial spoke of 1. For radial spokes 2, 3, and 6, the average T1 and T2 values are in reasonable agreement with T1 and T2 values of prostate normal transition zone reported in literature (4).Discussion
Radial readout allows for substantially shorter repetition times (TR) compared with previously investigated spiral readout methods, which is expected to reduce sensitivity to B0 inhomogeneities compared with spiral readout methods. In this preliminary in-vivo work, we demonstrate the feasibility of radial MRF in the prostate. While reconstructed images exhibit high spatial integrity for all examined levels of acquisition speed, we demonstrated a trend in variation for T1 and T2 values in relation to level of undersampling. The mapping values seems to be confounded by the expected lower signal to noise ratio for accelerated acquisitions. In future work, we plan to explore combined iterative reconstruction methods (7) for further improvements in T1 and T2 mapping fidelity for accelerated acquisitions.Conclusion
Feasibility of high-resolution quantitative prostate parameter mapping with golden angle radial MR fingerprinting has been successfully demonstrated.Acknowledgements
This research work was performed in collaboration and under an institutional master research agreement with Philips Healthcare. This work was partially supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748). The authors would like to thank the MRI technologists and therapists in the MSKCC Department of Radiation Oncology for their skilled assistance.References
1. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495(7440):187-192.
2. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magnetic resonance in medicine 2015;74(6):1621-1631.
3. Yu AC, Badve C, Ponsky LE, Pahwa S, Dastmalchian S, Rogers M, Jiang Y, Margevicius S, Schluchter M, Tabayoyong W. Development of a combined MR fingerprinting and diffusion examination for prostate cancer. Radiology 2017;283(3):729-738.
4. Sushentsev N, Kaggie JD, Buonincontri G, Schulte RF, Graves MJ, Gnanapragasam VJ, Barrett T. The effect of gadolinium-based contrast agent administration on magnetic resonance fingerprinting-based T 1 relaxometry in patients with prostate cancer. Scientific reports 2020;10(1):1-9.
5. Li Z, Berman BP, Galons J-P, Bilgin A, Altbach MI, Martin DR. Rapid high-resolution T1 mapping using highly accelerated radial steady-state free-precession acquisition. 2016. p 4196.
6. Jiang Y, Ma D, Keenan KE, Stupic KF, Gulani V, Griswold MA. Repeatability of magnetic resonance fingerprinting T1 and T2 estimates assessed using the ISMRM/NIST MRI system phantom. Magnetic resonance in medicine 2017;78(4):1452-1457.
7. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden‐angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden‐angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine 2014;72(3):707-717.
Figures