1553
Cardiac motion-corrected image reconstruction for Cardiac Magnetic Resonance Fingerprinting1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany
Synopsis
Cardiac magnetic resonance fingerprinting (cMRF) is a promising framework for quantitative assessment of various cardiomyopathies. One major challenge is cardiac motion. The majority of cMRF methods use ECG triggering and gating to select only certain cardiac phases to reconstruct cMRF data. In this study, we propose an iterative motion-correction approach utilizing the entire cardiac MRF acquisition. Obtained results show an improvement in obtained maps and consistent quantifications of T1 and T2.
Introduction
Cardiac magnetic resonance fingerprinting (cMRF) is a promising tool for the assessment of cardiomyopathies because it allows for the quantification of both T1 and T2 in a single-breathhold scan1. One major challenge of cMRF is cardiac motion during the acquisition process.The majority of cMRF techniques use cardiac gating or triggering to minimize cardiac motion-induced artefacts1-3. Hamilton et al. proposed cardiac motion correction as a pre-processing step before pattern matching to utilise all acquired data for T1 and T2 estimation4. However, this requires the contrast changes during MRF data acquisition to be considered and that motion estimation and pattern matching have the same temporal resolution.
In order to overcome this limitation, we propose to integrate cardiac motion correction into the image reconstruction to decouple motion correction and pattern matching. This also ensures that the motion correction is constrained by the data consistency term of the image reconstruction.
Method
MRF Acquisitions: All acquisitions were performed on a 3T Verio MR Scanner (Siemens Healthineers, Erlangen, Germany) using a 32-channel cardiac coil (Siemens Healthineers, Erlangen, Germany). A FLASH-based sequence with golden-angle radial data acquisition was utilized. A varying flip-angle pattern which is robust to B1-inhomogeneities was used after a 21ms non-selective inversion pulse5. Data was acquired with the following parameters: Length of pulse train = 1500; FOV = 320*320mm2; Resolution = 1.3*1.3mm2; Fixed TE = 4ms; Fixed TR = 8.2ms; Acquisition time = 12s; Slice thickness = 8mm. To reduce the impact of the slice profile on parameter quantification, a bandwidth time product of 8 was used (SINC pulse length = 1920ms). ECG triggering is used to start the acquisition during diastole, but data is then acquired continuously without further triggering.MOLLI and a bSSFP sequence with T2-preparation pulses (T2-prep times: 0, 25, 55 ms ) were acquired with cardiac triggering in diastole and used as references for T1 and T2, respectively. In-vivo acquisitions were carried out in three healthy volunteers during a breathhold.
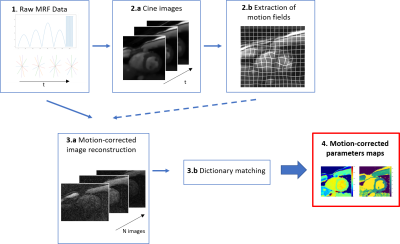
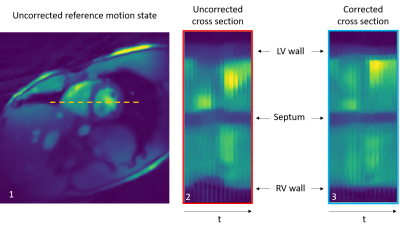
Motion Registration and correction: Figure 1 gives an overview of the proposed pipeline. In order to register and then correct for cardiac motion, cine images were first reconstructed from the cMRF acquisition. For this, the cMRF data was retrospectively binned into 15 cardiac phases based on the recorded ECG signal. Due to the incoherence between MRF contrast variation and cardiac cycle length, the contrast of all cardiac phases was similar which ensured robust image registration. Then, using the MIRTK toolkit6, motion fields were extracted and applied during MRF image reconstruction7. Figure 2 shows an example of the cine images before and after motion correction to highlight the effect of cardiac motion correction.
MRF Image Reconstruction: A sliding-window approach was implemented to accelerate the reconstruction process8. Instead of reconstructing 1500 images, each with one radial line, 741 images with 20 projections each were reconstructed. This process enables a faster reconstruction process without any information loss8. A conjugate gradient reconstruction with 16 iterations was used. Previously obtained motion fields were applied at each iteration of the image reconstruction to directly obtain cardiac-motion corrected images9.
Dictionary calculation and matching: An Extended-Phase-Graph based dictionary was used with the following input parameters : T1 = (200-3000ms, 14ms increments) ; T2 = (20-150ms, 0.65ms increments)10. The simulated fingerprints were then matched with the acquired data to obtain T1 and T2 maps. In order to realize the matching, dot-products between theoretical fingerprints and the cMRF data were calculated. The theoretical fingerprint with the highest dot-product value is considered as the best match.
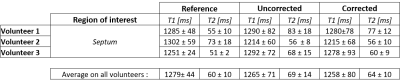
Mapping evaluation: To evaluate the obtained maps, a region of interest (ROI) was drawn on the septum. T1/T2 mean values and standard deviations were then calculated for the references (MOLLI and T2-prep bSSFP), the uncorrected cMRF acquisition and the cardiac motion-corrected cMRF acquisition in the specified ROI.
Results and discussion
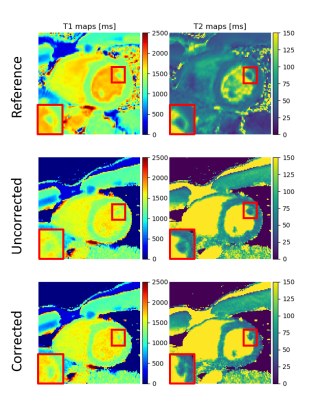
Figure 3 compares the uncorrected and cardiac motion-corrected T1/T2 maps to the reference acquisitions. While the uncorrected maps show already high image quality resulting from the radial type of acquisition11, the in-plane motion corrected relaxometric maps reflect differences particularly in the smaller anatomical details such as papillary muscles.Table 1 compares the T1/T2 values measured in the septum to the reference measurements. Motion correction has little effect on T1 quantification and leads to a better agreement with the cardiac-triggered reference measurements for T2. T1/T2 values are consistent with literature12 albeit with a small overestimation of T2 (literature: 45ms12, our approach: 64ms). This could be due to B1+ inhomogeneities which will require further investigation. So far, we also have not analyzed the effect of through-plane motion on cMRF quantification which will also be part of a future work.
Conclusion
This work presented the implementation of an iterative motion correction reconstruction to directly obtain cardiac motion-corrected images for cMRF quantification. We could demonstrate in-vivo that this approach improves anatomical visualization. Further work is required to improve T2 quantification.Acknowledgements
The results presented here have been developed in the framework of the 18HLT05 QUIERO Project. This project has received funding from the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.References
[1] Y. Liu, J. Hamilton, S. Rajagopalan, N. Seiberlich, Cardiac magnetic resonance fingerprinting: technical overview and initial results, JACC Cardiovasc. Imaging, 11 (12) (2018), pp. 1837-1853, 10.1016/j.jcmg.2018.08.028
[2] Cruz G, Jaubert O, Botnar RM, Prieto C. Cardiac Magnetic Resonance Fingerprinting: Technical Developments and Initial Clinical Validation. Curr Cardiol Rep. 2019 Jul 27;21(9):91. doi: 10.1007/s11886-019-1181-1.
[3] Hamilton JI, Jiang Y, Ma D, Lo WC, Gulani V, Griswold M, Seiberlich N. Investigating and reducing the effects of confounding factors for robust T1 and T2 mapping with cardiac MR fingerprinting. Magn Reson Imaging. 2018 Nov;53:40-51. doi: 10.1016/j.mri.2018.06.018.
[4] Hamilton JI, Jiang, Y, Eck, B, Griswold, M, Seiberlich, N. Cardiac cine magnetic resonance fingerprinting for combined ejection fraction, T1 and T2 quantification. NMR in Biomedicine. 2020; 33:e4323. https://doi.org/10.1002/nbm.4323
[5] Buonincontri, G. and Sawiak, S.J. (2016), MR fingerprinting with simultaneous B1 estimation. Magn. Reson. Med., 76: 1127-1135. https://doi.org/10.1002/mrm.26009
[6] D. Rueckert, L. I. Sonoda, C. Hayes, D. L. G. Hill, M. O. Leach and D. J. Hawkes, "Nonrigid registration using free-form deformations: application to breast MR images," in IEEE Transactions on Medical Imaging, vol. 18, no. 8, pp. 712-721, Aug. 1999, doi: 10.1109/42.796284.
[7] Batchelor, P.G., Atkinson, D., Irarrazaval, P., Hill, D.L.G., Hajnal, J. and Larkman, D. (2005), Matrix description of general motion correction applied to multishot images. Magn. Reson. Med., 54: 1273-1280. https://doi.org/10.1002/mrm.20656
[8] Cao, X., Liao, C., Wang, Z., Chen, Y., Ye, H., He, H. and Zhong, J. (2017), Robust sliding‐window reconstruction for Accelerating the acquisition of MR fingerprinting. Magn. Reson. Med, 78: 1579-1588. https://doi.org/10.1002/mrm.26521
[9] Pruessmann, K.P., Weiger, M., Börnert, P. and Boesiger, P. (2001), Advances in sensitivity encoding with arbitrary k‐space trajectories. Magn. Reson. Med., 46: 638-651. https://doi.org/10.1002/mrm.1241
[10] Weigel, M. (2015), Extended phase graphs: Dephasing, RF pulses, and echoes ‐ pure and simple. J. Magn. Reson. Imaging, 41: 266-295. https://doi.org/10.1002/jmri.24619
[11] Becker, KM, Blaszczyk, E, Funk, S, et al. Fast myocardial T1 mapping using cardiac motion correction. Magn Reson Med. 2020; 83: 438– 451. https://doi.org/10.1002/mrm.27935
[12] von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, Niendorf T, Schulz-Menger J. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson. 2013 Jun 18;15(1):53. doi: 10.1186/1532-429X-15-53.
Figures