1552
Myocardial T1, T2, T2* and Fat Fraction Quantification via Low-Rank Motion-Corrected Cardiac MRF1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Cardiac Magnetic Resonance Fingerprinting (MRF) has been proposed for simultaneous myocardial T1, T2 and fat fraction quantification using ECG-triggering, mid-diastolic acquisition window and a 3-echo gradient echo sequence. Here we extend this framework to further enable T2* quantification. This is achieved with an 8-echo sequence with increased acquisition window (to acquire sufficient data within a breath-hold) and a low-rank motion correction reconstruction to correct for cardiac motion within this increased window. The proposed approach enables simultaneous mapping of T1, T2, T2* and fat fraction within a single breath-hold with similar quality to conventional (sequential) single parameter approaches.
INTRODUCTION:
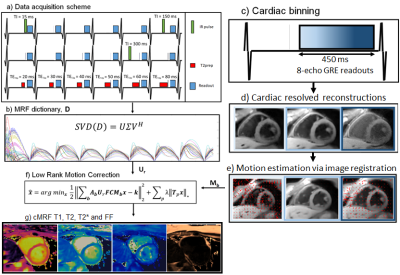
Cardiac Magnetic Resonance Fingerprinting (MRF)1 has been proposed for simultaneous myocardial T1, T2 and fat fraction (FF) tissue characterization.2 This approach uses ECG-triggering to synchronize data acquisition to a small mid-diastolic window (~200ms), reducing cardiac motion artefacts but also limiting the amount of acquired data per heartbeat. Increasing the acquisition window can improve scan efficiency, allowing the mapping of additional relevant parameters, however resulting in increased sensitivity to cardiac motion. Here we propose an eight-echo gradient echo sequence with large acquisition window combined with a Low Rank Motion Corrected (LRMC)3,4 reconstruction to correct for cardiac motion and enable simultaneous T1, T2, T2* and FF maps in a single breath-hold scan. LRMC combines elements of low rank modelling5,6,7 (to resolve contrast) and dense motion fields8,9,10 (for non-rigid motion correction). Preliminary evaluation of the proposed approach was performed in five healthy subjects in comparison to non-motion corrected low rank MRF and the corresponding conventional mapping techniques.METHODS:
Data acquisition follows a similar design to previous T1, T2 and FF cardiac MRF,2 relying on inversion recovery (IR) and T2 preparation (T2prep) prepulses in different heartbeats and multiple echo times per excitation (Fig.1a). In contrast to previous approaches, eight echoes are acquired per excitation with an increased cardiac acquisition window of 480 ms (Fig.1c). The reconstruction framework employed can be split in three steps: 1) estimate dictionary-based low rank compression basis $$$U_r$$$ (Fig.1b), 2) estimate non-rigid cardiac motion $$$M_n$$$ (Fig.1d-e), 3) perform LRMC reconstruction (Fig.1f). $$$U_r$$$ is obtained from a truncation of the left singular vectors following a singular value decomposition of the MRF dictionary. To estimate motion, a preliminary motion resolved reconstruction is performed with LRI-HDPROST11 after binning the data into multiple cardiac phases:$$ L(y_n , T_b^n)= argmin_{y_n , T_b^n} ||A_nU_rFCy_n-k_n||_2^2 + \lambda Σ_b ||T_b^n||_* , s.t. T_b^n=Q_b(y_n)$$
where $$$y_n$$$ are the reconstructed singular images for the n-th motion state, $$$A_n$$$ corresponds to k-space sampling, $$$U_r$$$ is the low rank compression, $$$F$$$ is the non-uniform Fourier transform, $$$C$$$ are the coil sensitivities, $$$k_n$$$ is the k-space and $$$Q_b$$$ generates the 3D HD-PROST tensor. Cardiac motion is estimated from $$$y_n$$$ via image registration12 and incorporated into the LRMC reconstruction:
$$ L(y , T_b)= argmin_{y , T_b} ||Σ_nA_nU_rFCM_ny-k||_2^2 + \lambda Σ_b ||T_b||_* , s.t. T_b=Q_b(y)$$
where $$$y$$$ are motion corrected singular images and $$$M_n$$$ are the motion fields. Resulting singular images are water/fat separated using a graph cut scheme13. Subsequently, water separated singular value images are used in standard MRF dictionary matching to obtain water-specific T1 and T2; the same graph cut scheme is employed to obtain T2* and FF, using the first singular image.
EXPERIMENTS:
Five healthy subjects were scanned at a 1.5T scanner (Philips Ingenia) with the proposed approach. Imaging parameters included field of view (FOV) = 256x256 mm2; 8 mm slice thickness; resolution = 2x2 mm2; TE1/ΔTE/TR = 1.6/1.8/16 ms; eight-echo gradient echo readout; flip angle 15º; acquisition window = 480 ms; 540 time-points; nominal scan time 18s. LRMC considered 10 cardiac phases for motion correction and r = 8. Conventional MOLLI, T2-GraSE, eight-echo gradient echo (for T2*) and six-echo gradient echo (for FF) were acquired for comparison. Acquired MRF data was reconstructed with the proposed LRMC and with no motion correction (NMC) via LRI-HDPROST.RESULTS:
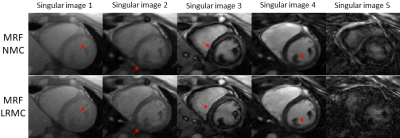
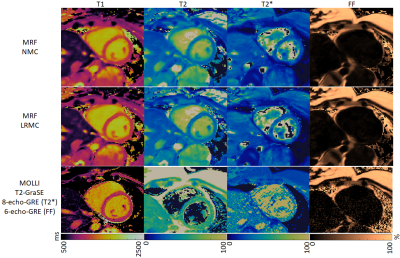
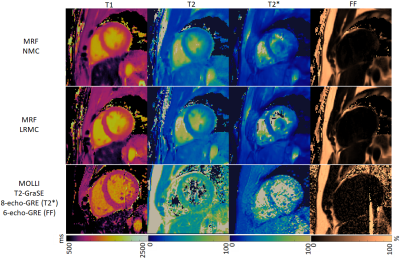
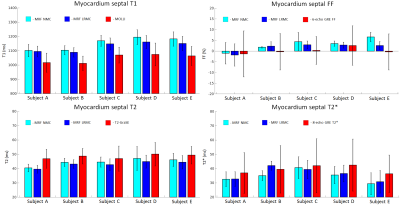
Due to the long cardiac acquisition window, residual blurring artefacts can be observed in the reconstructed singular images, which are considerably reduced after motion correction via LRMC (Fig.2). With NMC, the artefacts propagate into the final parametric maps, blurring the myocardium and papillary muscles; however, motion artefacts are corrected with LRMC leading to comparable quality to conventional maps (Fig.3 and Fig.4). Parameter values in the septal wall are shown in Figure 5 where cardiac MRF estimates slightly higher T1 values than MOLLI, slightly lower T2 and T2* than T2-GraSE and 8-echo gradient echo (respectively), and similar values for FF compared to 6-echo gradient echo. Mean septal values of the cohort for NMC MRF, LRMC MRF and corresponding conventional methods were 1150ms, 1129ms and 1048ms for T1; 44.5 ms, 43.0 ms and 48.2ms for T2; 34.6 ms, 34.7ms and 39.4ms for T2*; 3.1%, 1.6% and 0.3% for FF. Corresponding mean septal spatial variability (measured via standard deviation) of the cohort were 43ms, 41ms and 64ms for T1; 4.4ms, 3.8ms and 7.0ms for T2; 6.0ms, 5.7ms and 16.2ms for T2*; 2.6%, 2.4% and 8.7% for FF.CONCLUSION:
Simultaneous, multi-parametric, co-registered T1, T2, T2* and fat fraction estimation is achieved with cardiac MRF, by use of long cardiac acquisition windows and a novel Low Rank Motion Corrected reconstruction to correct cardiac motion. Parameter maps with comparable quality to conventional approaches are obtained in a single breath-hold. When performed pre- and post-contrast, the proposed approach could provide all the currently recommended parameters for myocardial tissue characterization14 from a single scan and will be further investigated in patients in future work.Acknowledgements
This work was supported by EPSRC (EP/P001009, EP/P032311/1, EP/P007619/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T 1 , T 2 , and proton spin density. MRM 2017;77:1446–1458.
2. Jaubert O, Cruz G, Bustin A, Schneider T, Botnar RM, Prieto C. Dixon-cMRF : cardiac tissue characterization using three-point Dixon MR fingerprinting. 2019:5–7 doi: 10.1002/mrm.26216.2.
3. Cruz G, Qi H, Jaubert O, Bustin A, Kuestner T, Schneider T, Botnar RM, Prieto C. Generalised Low-Rank Non-Rigid Motion Corrected reconstruction for 2D cardiac MRF. ISMRM 2020; abstract number 872.
4. Cruz G, Jaubert O, Qi H, Botnar RM, Prieto C. Generalised Low-Rank Non-Rigid Motion Corrected reconstruction for 3D free breathing liver MRF. ISMRM 2020; abstract number 3737.
5. McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans. Med. Imaging 2014;33:2311–2322 doi: 10.1109/TMI.2014.2337321.
6. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
7. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
8. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 2005;54:1273–1280 doi: 10.1002/mrm.20656.
9. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
10. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine. 2016 Apr;75(4):1484-98.
11. Bustin A, Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn. Reson. Med. 2019:3705–3719 doi: 10.1002/mrm.27694.
12. Modat M, Ridgway G, Taylor Z, Lehmann M, Barnes J, Hawkes D, Fox N, Ourselin S. Fast free-form deformation using graphics processing units. Comput Meth Prog Bio 2010; 98: 278– 284.
13. Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63:79-90.
14. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. Journal of Cardiovascular Magnetic Resonance. 2020 Dec;22(1):1-8.
Figures