1544
3D Ultrashort Echo Time MR Fingerprinting (3D UTE-MRF) for Whole Brain Myelin Imaging1Center for Brain Imaging Science and Technology, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, China, 2MR Collaboration, Siemens Healthcare Ltd, Shanghai, China, 3Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 4Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
Synopsis
We proposed a 3D ultrashort echo time MR fingerprinting (3D UTE-MRF) method for whole brain myelin imaging in vivo and ex vivo. A series of dual-echo time images were acquired, and images optimized for long T2 tissue suppression were found in the second echo image series, which were used to extract myelin signals. Non-negative least-square was used to map the ultrashort T2 myelin tissue fraction. 3D UTE-MRF sequence can achieve whole brain myelin mapping in 15 min with 0.87 mm isotropic resolution.
Introduction
Myelin mapping is of high importance in evalution of demyelination diseases. However, non-aquatic myelin proton is difficult to detect due to its ultrashort T2 relaxation 1. Recently, adiabatic inversion recovery ultrashort echo time (IR-UTE) 2 with TE less than 50 $$$\mu s$$$ has been proposed for direct myelin imaging in WM. However, previous studies 3,4 have shown that accurate TI is critically important for suppressing long T2 tissues. Even a slight TI offset will introduce significant contamination of other tissues. Advanced UTE techniques 3,4 have been proposed to mitigate these problems. However, these techniques take relative long scan time for whole brain imaging, difficult for routine clinical use. To address the aforementioned problems, we propose a 3D ultrashort echo time MR fingerprinting (UTE-MRF) technique for accurate and time efficient myelin imaging by dictionary matching.Methods
Experiments were performed on a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany) using a 64-channel head coil for both ex vivo and in vivo studies.Ex vivo brain imaging was performed on a formalin fixed single hemisphere human brain obtained from China Brain Bank (Zhejiang University School of Medicine). In vivo brain imaging was performed on two healthy volunteers (Subject 1: 23-year-old male; Subject 2: 28-year-old male). Informed consent was obtained from the donor before his death and from the healthy subjects.
A 3D UTE-MRF sequence we developed previously 5 was used in this study. The sequence diagram is shown in Fig. 1. With an adiabatic IR pulse, long T2 components are inverted while the short T2 myelin is mostly saturated by the long IR pulse. Following IR pulse, a train of center-out dual-echo acquisition was used in the MRF unit, where the flip angles (FA) varied from 5° to 60°. The first TE was minimized to 0.05 $$$ms$$$ and the second TE was set as 2.91 $$$ms$$$. TR was fixed to 7 $$$ms$$$. Total scan time was about 15 min for whole brain imaging. k-Space spokes at the same frame were reconstructed into a $$$220\times220\times220$$$ matrix under the resolution of $$$0.87\times0.87\times0.87$$$ $$$mm^3$$$ using NUFFT.
With the IR pulse and multiple RF pulses during the MRF unit, longitudinal magnetization of long T2 tissue recovered from inverted state while myelin recovered from saturated state. When long T2 tissue in WM reached null point, myelin contrast in WM was enhanced by echo subtraction (demonstrated in Fig. 2, replotted according to previous study 6). At the 1st TE, WM signal comes from positive short T2 tissue, while GM signal comes from both positive short T2 tissue and negative long T2 tissue. At the 2nd TE, short T2 tissue in both WM and GM fully decays, resulting in positive echo difference (echo1-echo2) in WM and negative one in GM. The optimum frame for myelin imaging can be found when no signals in WM in the second echo images as previously reported3 where the long T2 tissue signal is nulled and the short T2 myelin signal has fully decayed.
To recover the highly under-sampled data from each frame, we enforced a low-rank constraint in the image domain with constraining the solution to the dictionary subspace proposed before 7.
In addition, to quantify non-aquatic myelin component, non-negative least-square (NNLS) method 8,9 was used to decompose the MRF signals from the ultrashort echo.
For in vivo data, the four-compartment model includes CSF (T1 = 3000 $$$ms$$$, T2 = 2000 $$$ms$$$), GM (T1 = 1100 $$$ms$$$, T2 = 120 $$$ms$$$), WM (T1 = 800 $$$ms$$$, T2 = 70 $$$ms$$$), and non-aquatic myelin (T1 = 300 $$$ms$$$, T2 = 1 $$$ms$$$). The NNLS method was not performed on ex-vivo data, as there were no good approximates of relaxation values for myelin tissue.
Results
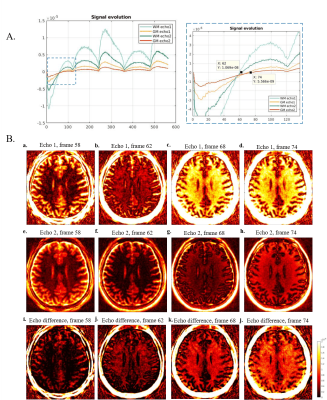
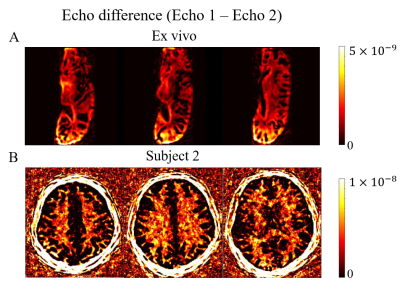
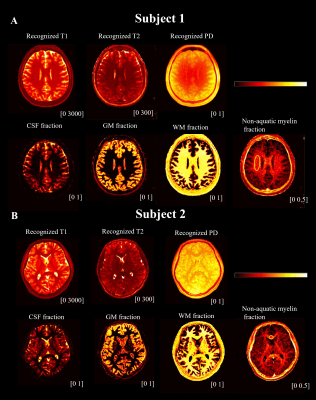
Fig. 3A shows the real part of the signal curves of both WM and GM from the dual-echo images (Subject 1). Frame 62 is recognized as the optimum frame for suppressing long T2 tissues in WM where the WM signals from the second echo time reached near zero. Enhanced myelin contrast is obtained from echo subtraction and shown in (j) of Fig. 3B. Fig. 4 shows the representative slices of myelin imaging acquired from both ex vivo human brain (A) and another healthy volunteer (Subject 2, B).Fig. 5 shows the recognized T1, T2, and PD maps from ultrashort echo MRF signals and NNLS results. Fig.5A shows the results from the same slice with Fig. 3. The non-aquatic myelin component can be detected and the corresponding myelin fraction of ROI (yellow circle in non-acquatic myelin proton tissue) is $$$0.25\pm 0.04$$$, which is in agreement with a previous MT study 10.
Discussion and Conclusion
The proposed 3D UTE-MRF sequence combines the advantages of UTE and MRF. UTE sequence with 50 $$$\mu s$$$ echo time can achieve non-aquatic myelin detection. Low-rank constraint in the image domain with dictionary subspace was used for better reconstruction of highly under-sampled dataset in each MRF frame. Application of two echoes in UTE-MRF helps to derive and visualize myelin contents. The sequence allows whole-brain non-aquatic myelin mapping at 0.87 $$$mm$$$ isotropic resolution in 15 min.Acknowledgements
No acknowledgement found.References
1. Waldman A, Rees JH, Brock CS, et al. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology. 2003; 45(12):887-892.
2. Du J, Ma G, Li S, et al. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. NeuroImage. 2014;87:32-41.
3. Ma Y, Searleman AC, Jang H, et al. Whole-brain myelin imaging using 3D double-echo sliding inversion recovery ultrashort echo time (DESIRE UTE) MRI. Radiology. 2020;294(2):362-374.
4. Ma Y, Jang H, Wei Z, et al. Myelin imaging in human brain using a short repetition time adiabatic inversion recovery prepared ultrashort echo time (STAIR UTE) MRI sequence in multiple sclerosis. Radiology. 2020; 200425.
5. Li Q, Cao X, Ye H, et al. 3D UTE-MRF for multiple parametric maps with sub-millimeter isotropic resolution using multi-dimensional golden-angle radial trajectory. in ISMRM 28th Annual Meeting. 2020. Virtual meeting.
6. Ma Y, Jang H, Chang EY, et al. Ultrashort echo time (UTE) magnetic resonance imaging of myelin: technical developments and challenges. Quantitative Imaging in Medicine and Surgery. 2020; 10(6): 1186-1203.
7. Mazor G, Weizman L, Tal A, et al. Low-rank magnetic resonance fingerprinting. Med. Phys. 2018;45(9):4066-4084.
8. Chen Y, Chen MH, Baluyot KR, et al. MR fingerprinting enables quantitative measures of brain tissue relaxation times and myelin water fraction in the first five years of life. NeuroImage. 2019;186:782-793.
9. Liao C, Wang K, Cao X, et al. Detection of lesions in mesial temporal lobe epilepsy by using MR fingerprinting. 2018; 288(3):804-812.
10. Saccenti L, Hagiwara A, Andica C, et al. Myelin measurement using quantitative magnetic resonance imaging: a correlation study comparing various imaging techniques in patients with multiple sclerosis. Cells. 2020;9(393):9020393.
Figures
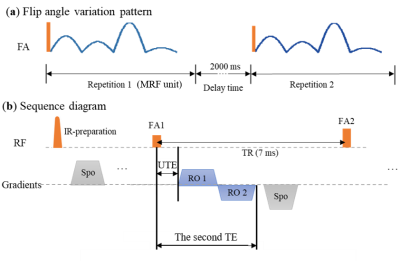
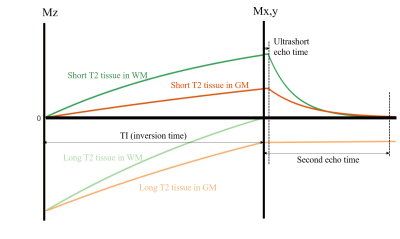
Fig. 2. This is replotted from paper 6.With IR pulse, short T2 tissue is saturated while the long T2 tissue is inverted. With a proper TI, long T2 tissue in WM is nulled. At the 1st TE, WM signal only comes from short T2 tissue in WM (myelin), while in GM, there is a cancellation between positive short T2 and negative long T2 tissue signals, leading to a net reduction in $$$M_x_y$$$. At the 2nd TE, short T2 tissues in both WM and GM has decayed, leading to a positive echo difference (echo1-echo2) in WM and negative one in GM.