1532
Development of a Clinical CEST-MR Fingerprinting (CEST-MRF) Pulse Sequence and Reconstruction Methods1Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiology, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Development of a CEST-MR Fingerprinting (CEST-MRF) pulse sequence combined with a physics-based deep learning approach suitable for use on a 3T clinical scanner is described and its utility demonstrated in a healthy human brain. The acquisition is short (less than 2 minutes) and simultaneously yields 6 quantitative tissue parameters that can be used for tissue characterization.
Introduction
Chemical Exchange Saturation Transfer (CEST) is a novel imaging technique that uses RF pulses to saturate the magnetization of exchangeable protons on proteins and metabolites [1]. CEST shows great promise for assessing disease pathologies, disease progression and therapeutic response in stroke and cancer [2,3], but suffers from technical limitations including long scan times and non-quantitative contrast. Recently, a preclinical CEST pulse sequence based on the MR Fingerprinting (MRF) framework was described and shown to yield quantitative exchange maps in the mouse brain [4,5]. The goal of this work is to adapt the CEST-MRF sequence to clinical scanners to enable in vivo human imaging in clinically acceptable scan times. We demonstrate the feasibility of simultaneous quantification of 6 relaxation and exchange parameters in clinically acceptable (<2 minutes) scan times on a 3T scanner.Methods
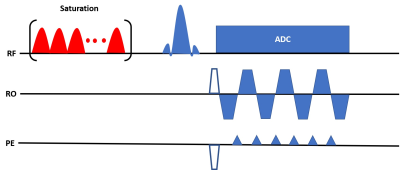
Pulse SequenceAll experiments were conducted on a Signa Premier 3T scanner (GE Healthcare, Waukesha, WI) with a 48-channel head receiver coil. The proposed pulse sequence diagram is shown in Figure 1. Hardware limits on clinical systems preclude the use of a continuous saturation pulse so a saturation pulse train was used instead. The pulse train consisted of 160 Gaussian shaped pulses of 16 ms duration centered on the amide proton frequency of 3.5 ppm. The saturation power was varied according to the schedule shown in Figure 2. The repetition time (TR), excitation flip angle (FA) and saturation duration (Tsat) were as follows: TR=3500 ms, FA=90°, Tsat=2560 ms. An EPI readout with partial Fourier factor of ~6/8 and parallel imaging acceleration factor of 3 resulted in an echo time (TE) of 24 ms. The matrix size was 224×224 with a FOV of 280 mm2 for an in-plane resolution of 1.25 mm2 and a slice thickness of 5 mm. The acquisition of the 30 schedule time steps required 105 seconds.
Physics-Based Deep Learning Tissue Parameter Quantification
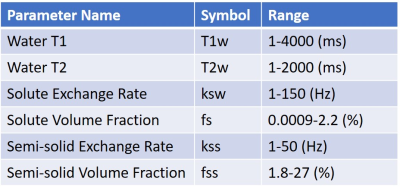
Dictionary matching for multiple parameters requires impractically large dictionaries. Instead, a DRONE neural network [6] with 4 hidden layers and 300 nodes per layer was used. The network was trained with a 500,000 entries simulated dictionary of signal magnetizations that was generated using the parameter ranges shown in Table 1. The signal magnetizations were calculated by solving the 3-pool (water, solute, semi-solid) Bloch-McConnel equations for each set of tissue parameters in the dictionary. The network outputs were the quantitative water T1 and T2 relaxation parameters (T1w, T2w) as well as the 4 quantitative CEST parameters: solute exchange rate (ks), volume fraction (fs), semi-solid exchange rate (kss) and volume fraction (fss). The network was trained to convergence using the ADAM optimizer [7] with a batch size of 1000, learning rate of 0.0001 and the L1 loss function.
In Vivo Human Scan
A healthy, 31 years old female volunteer was recruited and gave informed consent in accordance with our institution’s IRB protocol. The subject was scanned with the proposed CEST-MRF sequence and the data reconstructed as described.
Results
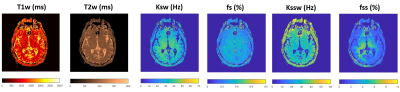
The proposed CEST-MRF sequence yields simultaneous relaxation and exchange tissue maps at clinical resolutions (~1mm) in a short scan time (less than 2 minutes) (Figure 3). Mean gray and white matter values for the different tissue parameters are shown in Table 2. The tissue parameter values obtained correspond well to known values from the literature [8–10].Discussion/Conclusion
The combination of MRF encoding and physics-based deep learning signal matching enables robust quantification of relaxation and exchange parameters. The reconstructed values were not corrected for B1 or B0 inhomogeneities. Correction for B1 and B0 is likely to improve the accuracy of the reconstructed tissue parameter maps and can be accomplished by means of a separate acquisition or by inclusion of B1 and B0 in the training dictionary. Unlike dictionary matching, the DRONE training dictionary was sparsely sampled relative to the dimensionality of the parameter space and yielded continuous valued maps. The schedule used in this work was similar to [2], which was not optimized. Optimization of the schedule [11–14] can potentially improve discrimination between tissues or further reduce scan time. Future work will include testing the proposed sequence in disease subjects and incorporating simultaneous multi-slice techniques.Acknowledgements
No acknowledgement found.References
1. Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, Mu L, Nanz D, Tuura R, Delso G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. Springer; 2016;3(1):19.
2. Ma B, Blakeley JO, Hong X, Zhang H, Jiang S, Blair L, Zhang Y, Heo H-Y, Zhang M, van Zijl PC, others. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J Magn Reson Imaging. 2016;44(2):456–462.
3. Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu D-X, Ford E, others. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. Nature Publishing Group; 2011;17(1):130–134.
4. Cohen O, Huang S, McMahon MT, Rosen MS, Farrar CT. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn Reson Med. Wiley Online Library; 2018;80(6):2449–2463.
5. Perlman O, Ito H, Herz K, Shono N, Nakashima H, Zaiss M, Chiocca EA, Cohen O, Rosen MS, Farrar CT. AI boosted molecular MRI for apoptosis detection in oncolytic virotherapy. bioRxiv. Cold Spring Harbor Laboratory; 2020;
6. Cohen O, Zhu B, Rosen MS. MR fingerprinting deep reconstruction network (DRONE). Magn Reson Med. 2018;80(3):885–894.
7. Kingma DP, Ba J. Adam: A method for stochastic optimization. ArXiv Prepr ArXiv14126980. 2014;
8. Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med Off J Int Soc Magn Reson Med. Wiley Online Library; 2005;54(3):507–512.
9. Heo H-Y, Han Z, Jiang S, Schär M, van Zijl PC, Zhou J. Quantifying amide proton exchange rate and concentration in chemical exchange saturation transfer imaging of the human brain. Neuroimage. Elsevier; 2019;189:202–213.
10. Van Zijl PC, Zhou J, Mori N, Payen J-F, Wilson D, Mori S. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med Off J Int Soc Magn Reson Med. Wiley Online Library; 2003;49(3):440–449.
11. Cohen O, Rosen MS. Algorithm comparison for schedule optimization in MR fingerprinting. Magn Reson Imaging. 2017;41:15–21.
12. Cohen O. MR Fingerprinting Schedule Optimization Network (MRF-SCONE). Proc Int Soc Magn Reson Med. Montreal; May 11-May 16. p. 4531.
13. Perlman O, Farrar CT, Cohen O. Deep Learning Global Schedule Optimization for Chemical Exchange Saturation Transfer MR Fingerprinting (CEST-MRF). Proc Int Soc Magn Reson Med. Virtual Conference; August 08-14. p. 3576. 14. Perlman O, Herz K, Zaiss M, Cohen O, Rosen MS, Farrar CT. CEST MR-Fingerprinting: practical considerations and insights for acquisition schedule design and improved reconstruction. Magn Reson Med. 2020;83(2):462–478.
Figures