1531
Rapid T1, T2 measurements and SNR evaluation by 31P MR fingerprinting in human brain at 7T
Song-I Lim1,2, Mark Stephan Widmaier1,3, Yun Jiang4, and Lijing Xin1,2
1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Laboratory for Functional and Metabolic Imaging, EPFL,, Lausanne, Switzerland, 4Department of Radiology, University of Michigan, Ann Arbor, MI, United States
1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Laboratory for Functional and Metabolic Imaging, EPFL,, Lausanne, Switzerland, 4Department of Radiology, University of Michigan, Ann Arbor, MI, United States
Synopsis
This study is to validate 31P MRS fingerprinting scheme at 7T. In this experiment, MRF scheme was validated using Pi phantom and in vivo brain data was acquired.
Introduction
Adenosine triphosphate (ATP) metabolism is the most fundamental process for supporting various cellular activities, which maintain the normal function and structural integrity of the brain. There is extensive evidence for abnormalities of brain bioenergetics in neurological diseases. Phosphorus MRS allows in vivo measurement of ATP metabolism, however, it suffers from its low sensitivity and long acquisition time. The magnetic resonance fingerprinting (MRF) technique is a novel quantitative and time-efficient measurements framework1. In a preclinical study2, it was already proven to be promising to measure multi-parameters in a short period of time. The purpose of the study is to develop a 31P MRS-FP (magnetic resonance spectroscopy fingerprinting) scheme at 7Tfor rapid relaxation and concentration measurement in the human brain.Methods
A balanced steady-state free precession type sequence was developed and implemented. The repetition time (TR) was fixed to 16 ms and a 1000-frame sinc-shaped slice selective excitation pulse train (2 cm slice thickness) with varying flip angles was applied, which is followed by a broadband inversion pulse (HS4; 10.24ms; inversion bandwidth = 4.9kHz; B1/2π = 1kHz) and 20 ms of inversion delay. Flip angle pattern consists of two parts as the following: 1) 760-frame of long sinusoidal shape and 2) 240-frame of drastic flip angle changes. The echo time was fixed and set so that the center of 10 ms of ADC is at the center of TR. Figure 1 briefly described the MRS-FP scheme used in this study. MR experiments were performed on a 7T/68cm MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 1H quadrature surface coil (10cm-diameter) and a single-loop 31P coil (7cm-diameter) designed for data acquisition from the occipital lobe. A phantom with 100 mM Pi and 16µM Gadovist was used to verify the feasibility of the MRSFP sequence. Inversion recovery method and sSPECIAL sequence based multiple TE method were used to acquire T1 and T2 relaxation times for the phantom and to compare with the results of MRS-FP scheme. After the conventional T1 and T2 measurements, a novel MRSFP method was applied to measure the relaxation times in phantom. In vivo data was acquired from a healthy subject (female; age = 40) who provided written informed consent.Results
Figure 2 shows the representative MRF-FP matching pattern. Table 1 shows the measured T1, T2 and γB1 values. Table 1 shows summarized results acquired using conventional 31P MRS acquisition and MRF-FP scheme. Figure 3 shows SNR evaluation to minimize the data acquisition time for further clinical application.Discussion and Conclusion
This is preliminary results to verify the feasibility of rapid and accurate MRF-FP scheme in clinical setting. In this study, the total measurement time was 8 min 40 seconds with 25 averages and 2 dummy scan. But Considering the SNR evaluation, it is expected to measure reliable data in 2 min.Acknowledgements
This work was supported by the Swiss National Science Foundation (grants n° 320030_189064). We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG)References
1. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–192 doi: 10.1038/nature11971.
2. Wang CY, Liu Y, Huang S, Griswold MA, Seiberlich N, Yu X. 31 P magnetic resonance fingerprinting for rapid quantification of creatine kinase reaction rate in vivo. NMR Biomed. 2017;30:1–14 doi: 10.1002/nbm.3786.
Figures
Figure 1 shows the MRS-FP pulse application scheme.
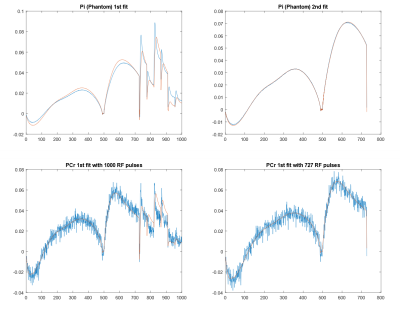
Figure 2 shows the representative MRF fitting using phantom and in vivo experiment.
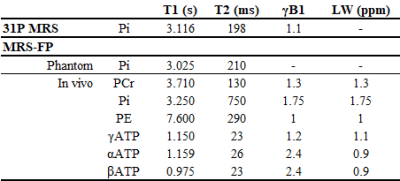
Table 1 shows the measured T1 and T2 relaxation time using MRS-FP scheme.
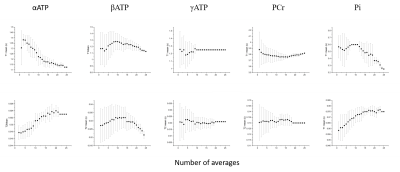
Figure 3 SNR evaluation for 5 measured metabolites in MRS-FP experiment. Scanning time can be shortened up to 2min without compensation of measurement reliability