1530
Influence of head motion on the output of Independent Component Analysis (ICA)-based denoising of task-related fMRI data at 7T1Human Brain Research Center, Kyoto University, Kyoto, Japan
Synopsis
Head motion during the acquisition of fMRI data can significantly contaminate the neural signal and induce spurious, distance-dependent changes in signal correlations. Framewise displacement (FD) has often been used as a cut-off threshold for removing bad fMRI datasets related with high motion. Here we investigated the influence of head motion on the output of ICA-based denoising analysis. The results showed a strong correlation between the number of total ICA components with head mean motion and FD, which indicated that the number of ICA components can be an index for detecting high motion-related fMRI datasets.
INTRODUCTION
Movement of the head during scans causes undesirable temporal fluctuations (i.e., noise), which make the identification of the effects that are truly related to the underlying neural activities (i.e., signal) difficult.1. Recently, ICA-based denoising technique has widely been used as a powerful tool for removing spurious motion-related signal from the data2. Furthermore, the framewise displacement (FD) variable, which measures movement of any given frame relative to the previous frame, has been used to regress out the head motion artifacts3,4 and to standardize a cut-off threshold for excluding high motion-related fMRI datasets5,6. However, the relationship between FD and ICA analysis has not been much elucidated. By investigating the influence of head motion on the output of ICA-based denoising, the present study aims to clarify their relationships.METHODS
Twenty-four right-handed young healthy subjects (13 males and 11 females, aged 20 - 25 years), who had no known history of neuropsychiatric disorders or substance abuse, participated in this study. All participants signed written informed consents under an IRB-approved protocol.In order to increase the possibility for observation of head movements during scans, we used motor tasks. Block-designed fMRI was used. Each fMRI run lasted for 6min30sec, consisting of 7 rest blocks alternatively presented with 6 task blocks. The subjects performed left hand grip motor task at 1Hz frequency during task blocks. Two consecutive fMRI runs of the same task condition were collected from all subjects (test, re-test paradigm). Scans were performed with a 7T whole-body scanner (MAGNETOM 7T, Siemens Healthineers, Erlangen, Germany) using a single-channel transmit and 32-channel receive head coil (Nova Medical, MA, USA). For functional volumes, multiband EPI sequence developed at CMRR7 with the following parameters was acquired: TR/TE = 1000/22 ms; FA = 45°; Multiband =5, GRAPPA = 2, spatial resolution = isotropic 1.6 mm. After acquiring the functional images, a whole-brain MP2RAGE anatomical images were acquired (TR/TE/TI1/TI2 = 6000/2.9/800/2700 ms; FA1/FA2 = 4/5°, spatial resolution = isotropic 0.7 mm).
The fMRI dataset was preprocessed by using a default preprocessing pipeline of HCP pipeline protocol (https://github.com/Washington-University/HCPpipelines). Motion correction of EPI images was processed with spatial realignment via MCFLIRT toolbox which is implemented within the default HCP pipeline protocol. The value of absolute root mean squared (RMS) movement of each volume, which describes motion of that volume with respect to the middle volume in time-series, was extracted from MCFLIRT report. The mean motion of an fMRI run is defined as the mean absolute RMS movement of all volumes of that scan. The number of fMRI volumes in which head motion was more than 0.2 mm and 0.5 mm different from adjacent volumes was also quantified4. Motion related fluctuation and different sources of artifacts were removed from the images using FMRIB’s ICA-based X-noiseifier (FIX) denoising analysis8. The output results of ICA-FIX analysis were re-confirmed by visual inspection of each ICA component9 from 2 researchers in the field.
RESULTS
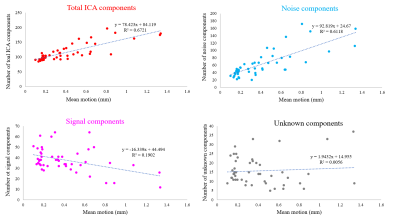
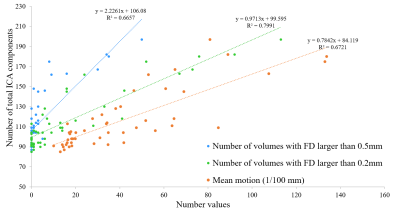
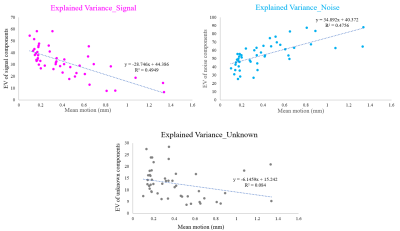
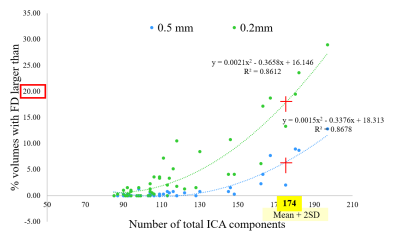
For each fMRI run, the average number of total ICA components was 116.2 (SD = 29) and the average numbers of signal/noise/unknown components were 37.8/62.6/15.8 (32.5/53.9/13.6 %), respectively. These results were re-confirmed by manual labelling, which showed that variance was less than 10% for each component classification (data are not shown).The total number of ICA components showed a strong correlation with mean motion (Figure 1) and FD (Figure 2). Increase in mean motion resulted in increase and decrease in explained variance of noise and signal components, respectively (Figure 3). The percentage of volumes with FD larger than 0.2mm significantly increased and nearly reached 20% where datasets are occasionally discarded, when the number of ICA components exceeded the value of mean + 2SD (Figure 4).
DISCUSSION
Head movements are well-established as one of the reasons for undesirable fluctuations in fMRI study. The acquisition of BOLD signal depends on precise spatial and temporal placement of magnetic gradients. Movement of the head during scans not only shifts the position of the brain in space but also disrupts the establishment of temporal readout of the BOLD signal1,10 resulting in spurious motion-related signal in the data. Therefore, evaluating and correcting head motion of an fMRI dataset is crucial. The fact that the number of total ICA components has a strong correlation with both mean motion and FD variables suggested that ICA decomposition can detect the spatial changes of the head throughout the fMRI scan, and the output results (i.e., the number of ICA components) indicated how much the subject moved during the scan.CONCLUSION
The number of total ICA components from ICA-based denoising analysis can be used as an index for evaluating and detecting high motion-related fMRI datasets.Acknowledgements
A research grant from Siemens Healthcare K.K., Japan. We also thank Yuta Urushibata from Siemens Healthcare K.K., Japan, for the helpful discussion and data analysis.References
1.Friston KJ, et al. Movement-related effects in fMRI timeseries. Magn Res Med. 1996; 35:346–355.
2.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004; 23:137–152.
3.Power JD et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012; 59(3):2142-54.
4. Power JD et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014; 84:320–341.
5. Fujiwara H et al. Neural correlates of non-clinical internet use in the motivation network and its modulation by subclinical autistic traits. Front. Hum. Neurosci. 2018; 10(12): 493.
6. Kobayashi K et al. Relationship between media multitasking and functional connectivity in the dorsal attention network. Sci Rep. 2020; 10(1):17992.
7. Moeller S et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010; 63(5):1144-1153.
8. Griffanti L, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 2014; 95:232–247.
9. Griffanti L, et al. Hand classification of fMRI ICA noise components. Neuroimage 2017; 154:188-205.
10. Hutton C et al. 2002. Image distortion correction in fMRI: a quantitative evaluation. NeuroImage 2002, 16: 217–240.