1524
Correlations analysis between changes in longitudinal ALFF and ReHo values of methamphetamine abstinence subjects and behavioral tests BIS-11
Yanyao Du1, Ru Yang1, Wenhan Yang1, Huiting Zhang2, and Jun Liu1
1Department of Radiology, the Second Xiangya Hospital of Central South University, Changsha, China, 2MR Scientific Marking, Siemens Healthcare Ltd., Wuhan, China
1Department of Radiology, the Second Xiangya Hospital of Central South University, Changsha, China, 2MR Scientific Marking, Siemens Healthcare Ltd., Wuhan, China
Synopsis
This study aimed to evaluate the correlation of longitudinal changes of amplitude low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) values based on Harvard-Oxford atlas (HOA) and Barratt Impulsivity Scale 11 (BIS-11) in subjects with methamphetamine abstinence. The results suggested that there were significant positively correlations between right middle frontal gyrus (8) and BIS total scores, BIS attention scores as well as BIS non-planning score.
Introduction
Methamphetamine (MA) is a highly addictive psychostimulant drug that can affect the central nervous system (CNS)1, 2, and becomes one of the most rapidly growing illicit drug. Previous study showed that MA abuse can cause comprehensive changes of brain structures and functions, but after a period of withdrawal, the brain function can be improved to a certain extent3. Amplitude low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) is two of the indicators that can represent the neuronal function changes of the regional brain area through blood oxygenation level-dependent (BOLD) technique, which is a function MRI (fMRI) method. Barratt Impulsivity Scale 11 (BIS-11) can measure the behavior and thinking patterns and reflect the neuronal function, and it is usually used in the clinical. In this study, we explored the correlation between changes of ALFF and ReHo values nearly one-year before and after abstinence and BIS-11 data in MA abstinence subjects who experienced short-term abstinence first and long-term abstinence later.Methods and materials
The study was approved by the ethics committee of our hospital. Written informed consent was obtained from all participants. 63 MA-dependent participants were recruited from drug rehabilitation centers. During abstinence, the participants were treated without MA, but with medicine, education, and physical exercise. 13 participants were excluded due to poor MR image quality due to severe head motions. Finally, 50 MA-abstinent right-handed people were included in this study. According to withdrawal time (<1 year or >1 year), they were divided into short-term abstinence group and long-term abstinence group. All imaging data were acquired on a 3T MRI scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. The MRI scanning included three-dimensional magnetically prepared rapid acquisition gradient echo (3D MPRAGE) sequence and resting-state fMRI sessions. The scan parameters were as follows: 3D MPRAGE, 176 sagittal slices, slice thickness=1 mm, gap=0 mm, FOV=256 mm×256 mm, TR=1450 ms, TE=2.03 ms, TI=900 ms, flip angle=30°, and voxel size=1×1×1mm3; resting-state fMRI, 36 axial slices, thickness=4 mm, FOV=220 mm×220 mm, TR=2000 ms, TE=30 ms, flip angle=80°, and 225 volumes. MR imaging preprocessing used the software named Data Processing Assistant for Resting-State fMRI (DPABI, 4.3, Advanced edition) (http://rfmri.org/dpabi). Furthermore, the whole-brain ALFF value of short-term and long-term abstinence participants were extracted separately from the HOA. The right middle frontal gyrus with significant difference ALFF and ReHo values between the short-term and long-term abstinence group was obtained using the paired sample t. Bivariate Pearson correlation analysis was applied to examine the associations between the right middle frontal gyrus in HOA and the BIS-11 data over the nearly one-year abstinence period.Results
Table 1 shows the demographic characteristics of the subjects. And there is no significant difference in the BIS-11 scores of the short-term and long-term abstinence groups (Table 2). It is right middle frontal gyrus (8) changes based on HOA in ALFF value (FDR-corrected P<0.005) between short-term and long-term abstinence group. In addition, the change in right middle frontal gyrus (8) based on HOA in ALFF value of long-term abstinence is positively correlated with BIS total scores (r=0.373, P=0.013), BIS attention scores (r=0.323, P=0.032) and BIS non-planning score (r=0.305, P=0.044) respectively (Figure 1). The change in right middle frontal gyrus based on HOA in ReHo value of long-term abstinence is positively correlated with BIS total scores (r=0.425, P=0.004), BIS attention scores (r=0.403, P=0.007) and BIS non-planning score (r=0.396, P=0.008) respectively (Figure 2).Discussion
Early study indicated that long-term MA use would cause damages to the function and self-control ability of the middle frontal gyrus, which is an important brain area inhibiting impulsivity, and result in the inability to suppress the craving for drugs4, 5. In our study, right middle frontal gyrus (8) had a significant positively correlation with BIS total scores, BIS attention scores and BIS non-planning score. Our experiment shows that for MA long-term abstinence subjects, non-planning subscale and attention subscales are more sensitive than motor, reflecting the decreased impulsivity. The study conducted by Winhusen et al. revealed that MA-dependents had significantly greater BIS-11 non-planning and total scores6. As far as our concern, no other study on MA long-term abstinence has reached a similar result, that subscales of different dimensions of BIS-11 have different results. That is to say, each item of each subscale may represent the 3 dimensions of the BIS-11, so the results of our study eventually showed that MA short-term abstinence are more impulse than MA long-term abstinence.Conclusion
This study found the right middle frontal gyrus had best positive correlation with BIS total scores, BIS attention scores and BIS non-planning score. Hence, the ALFF and ReHo based on HOA of the right middle frontal gyrus may be the potential imaging markers to reflect the change of function related to long-term withdrawal.Acknowledgements
No acknowledgement found.References
Figures
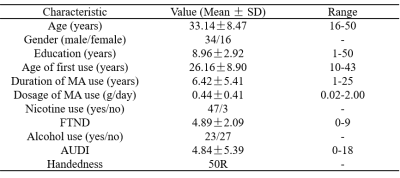
Table 1. Demographic characteristics of MA- abstinence subjects.
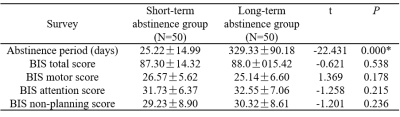
Table 2. BIS-11 data of short-term and long-term abstinence groups.
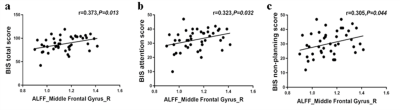
Figure 1. Correlations analysis between the changes in longitudinal ALFF values and the BIS-11 data.
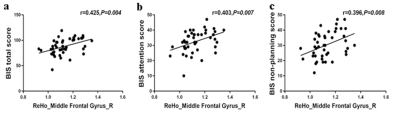
Figure 2. Correlations analysis between the changes in longitudinal ReHo values and the BIS-11 data