1516
Implementation of multi-contrast, multi-echo SAGE-fMRI in aging and Alzheimer’s disease1Division of Neuroimaging Research, Barrow Neurological Institute, Phoenix, AZ, United States, 2School of Life Sciences, Arizona State University, Tempe, AZ, United States, 3Division of Neurology, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
The purpose of this study was to apply a previously developed multi-contrast, multi-echo sequence using spin- and gradient-echo (SAGE) for fMRI in the context of Alzheimer’s disease (AD). We acquired pilot SAGE-fMRI data with five echoes (2 gradient-echoes, 2 asymmetric spin-echoes, 1 spin-echo) using episodic memory encoding and retrieval tasks in healthy aging and cognitively impaired (mild cognitive impairment (MCI) or mild AD) cohorts. Group differences in task-related activation were evaluated across single echo and multi-echo combined dynamic SAGE signals. SAGE-fMRI contrasts showed promise for more robust distinction between HC and CI groups than single-echo fMRI in AD-relevant brain regions.
Introduction
Functional MRI (fMRI) leverages the blood-oxygen-level dependent (BOLD) response to brain activity in order to map functional activation. Spin- and gradient-echo (SAGE), originally developed for perfusion imaging1,2, has recently been implemented in fMRI to overcome some of the challenges with standard fMRI methods, combining the advantages of multi-gradient-echo fMRI and spin-echo (SE) fMRI3,4. Single-echo fMRI suffers from susceptibility-induced signal dropout in areas with air-tissue interfaces, such as the medial temporal lobe and inferior frontal lobe, which is problematic for studying aging and memory function in these regions5,6. Multi-gradient-echo (GRE) fMRI improves upon single-echo acquisitions but still suffers from low spatial specificity5,6. SE fMRI improves spatial specificity but suffers from low BOLD sensitivity, resulting in lower contrast-to-noise ratio (CNR)7. SAGE combines the advantages of each of these methods, which is especially useful in the study of Alzheimer’s disease (AD), where the medial temporal lobe is vulnerable to AD pathology. Moreover, SAGE simultaneously provides macro- and micro-vascular signal sensitivity, enabling a more comprehensive assessment of vascular and functional changes in AD. We hypothesize that SAGE-based BOLD fMRI will improve BOLD signal in the medial temporal lobe, which will be leveraged in memory-related tasks to assess differences between healthy control (HC) and cognitively impaired (CI) groups.Methods
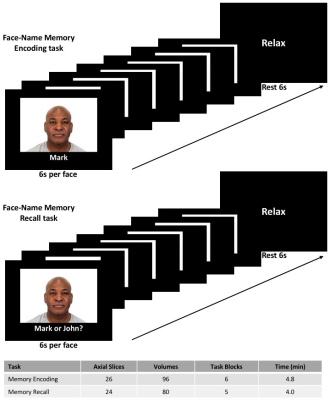
Five HC (4 females; 66.6±5.0 years) and four CI (mild cognitive impairment or mild AD; 3 females; 77.3±4.0 years) were included in this preliminary study. Each group underwent cognitive testing using the Montreal Cognitive Assessment8. MRI data were acquired at 3T (Ingenia, Philips); tasks were implemented using Presentation software. SAGE-fMRI data were acquired with 2 gradient-echoes, 2 asymmetric spin-echoes, and 1 spin-echo (TE1-5 = 5.97/18.76/36.048/49.27/62.06 ms, acquisition matrix: 64×64; voxel size: 3.75x3.75 mm; slice thickness: 5.0 mm; TR=3000 ms). For each TE, a reverse phase-encoding acquisition was collected to correct for EPI image distortions. The face-name memory encoding and recall tasks9 featured two conditions organized in alternating block designs and consisting of novel (i.e., unfamiliar faces) and repeated (i.e., two recurring faces) phases, with brief rest periods between conditions. Further acquisition parameters specific to each task can be found in Figure 1. SAGE-fMRI data were processed using single-echo and multi-echo combinations (GRE (macrovascular): TE2, T2*, wT2*; SE (microvascular): TE5, T2, wT2), where the w indicates weighted summations3. Specifically, TE2 and TE5 were processed individually, where TE2 is similar to standard fMRI acquisitions. Dynamic T2* and T2 were fit using a piece-wise function, while weighted summations for wT2* and wT2 were calculated as previously described3. Subsequently, all SAGE-fMRI images were processed using standard procedures with FSL and AFNI. AFNI was used to calculate the statistical parametric maps of response to stimuli from all fMRI data (3dDeconvolve) and to run a two-sample t-test between HC and CI groups (3dttest++).Results
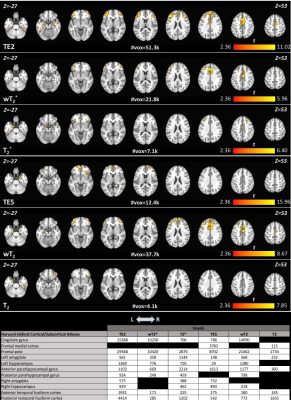
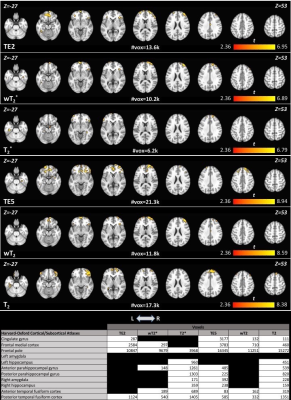
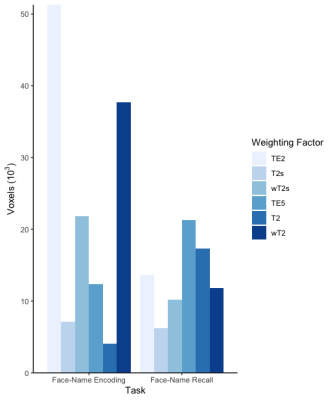
Differences between groups, for memory encoding and recall tasks across each SAGE-fMRI analysis method, are shown in Figures 2 and 3, where the HC group had more activation than CI across both tasks. Weighted summation SAGE contrasts showed robust differences between groups in the cingulate gyrus and frontal pole in the memory encoding task. Moreover, the frontal pole had sizeable differences between groups in memory recall, particularly in the microvascular contrasts; these weighting factors revealed more distinct differences between groups across regions of interest for the memory recall task than the macrovascular contrasts. Notably, in the memory recall task, T2* and T2 distinguished between groups in AD-relevant regions such as the hippocampus and parahippocampal gyrus where single-echo fMRI did not. Figure 4 shows the task-related voxel count differences (HC>CI) for the memory encoding and recall tasks across all SAGE-fMRI analysis methods. With the exception of TE2 in the memory encoding task, there are comparable voxel counts in SAGE-based contrasts to the single-echo contrasts.Discussion
These preliminary results support that SAGE-fMRI may more robustly distinguish differences between HC and CI groups, particularly in highly relevant regions to AD such as the hippocampus, medial temporal lobe, and inferior frontal lobe. Specifically, SAGE-fMRI may improve signal quantification in these regions vulnerable to susceptibility-induced signal dropout, as well as provide better spatial localization of functional activation due to its sensitivity to both macro- and micro-vasculature. Although prior simulation studies predicted a substantial CNR gain in AD-relevant regions3, these preliminary results on a small cohort showed only moderate changes in activation clusters. However, the lower voxel counts, particularly for T2* and T2, could indicate higher accuracy of measured task-related activation. Therefore, continuation of study enrollment will be important to further validate these preliminary results.Conclusion
SAGE-fMRI enables multi-echo, multi-contrast characterization sensitive to both macro- and micro-vasculature. By improving CNR, this method enables more accurate assessment of functional activation. Preliminary results showed widespread decrease in task-related activation from HC to CI groups, with further distinction between the two groups in AD-relevant regions such as the hippocampus and parahippocampal gyrus via SAGE contrasts. The benefits of SAGE, particularly the improved acquisition in the medial temporal and inferior frontal lobes, motivate its application in AD.Acknowledgements
This work was supported by the Barrow Neurological Foundation and Philips Healthcare.References
1. Stokes AM, Skinner JT, Quarles CC. Assessment of a combined spin- and gradient-echo (SAGE) DSC-MRI method for preclinical neuroimaging. Magn Reson Imaging. 2014;32(10),1181-90.
2. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2011;68(1),30-40.
3. Bergamino M, Steffes L, Gonzales A, et al. Optimization of vascular and functional sensitivity using multi-contrast, multi-echo SAGE-EPI for fMRI. Proc 28th Annu Meet ISMRM Sydney, Aust. 2020;5428.
4. Schmiedeskamp H, Peterson E, Maclaren J, et al. Multiband spin- and gradient-echo (SAGE) fMRI. Proc 22nd Annu Meet ISMRM, Milan, Italy. 2014;1503.
5. Kundu P, Brenowitz ND, Voon V, et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U.S.A. 2013;110(40):16187-92.
6. Posse S, Wiese S, Gembris D, et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn Reson Med.1999;42(1):87-97.
7. Binney RJ, Embleton KV, Jefferies E, et al. The Ventral and Inferolateral Aspects of the Anterior Temporal Lobe Are Crucial in Semantic Memory : Evidence from a Novel Direct Comparison of Distortion-Corrected fMRI , rTMS , and Semantic Dementia. Cereb Cortex 2010;20:2728–38.
8. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695-9.
9. Sperling RA, Bates JF, Cocchiarella AJ, et al. Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp. 2001;14(3):129-39.
Figures