1515
Evaluation and interaction of disease dependent head motion, low frequency oscillations, and cerebral blood flow in Alzheimer’s Disease cohort
Mu-Lan Jen1, Laura B. Eisenmenger2, Sterling C. Johnson3,4, Veena A. Nair2, Vivek Prabhakaran2, and Kevin M. Johnson1,2
1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, WI, United States, 4Wisconsin Alzheimer's Disease Research Center, University of Wisconsin-Madison, 53705, WI, United States
1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, WI, United States, 4Wisconsin Alzheimer's Disease Research Center, University of Wisconsin-Madison, 53705, WI, United States
Synopsis
Recent studies have suggested reduced altered low-frequency oscillations (LFOs) and cerebral blood flow (CBF) in subjects with Alzheimer’s disease (AD). These measures may reflect reduced neural activity and altered waste clearance but are subject to confounding effects from patient motion. This study of a cohort of AD and controls investigates interaction of patient motion with LFO from BOLD and CBF from multi-delay arterial spin labeling. Results demonstrate significantly increased motion in subjects with AD and a correlation of motion estimates with BOLD LFO but not with CBF. These results suggest motion should be considered for studies in neurodegenerative subjects.
Introduction
Alzheimer’s disease (AD) is associated with disruptions in brain function and altered hemodynamics.1,2 One measure that can offer insight into subtle functional and hemodynamic brain alterations are low frequency oscillations (LFOs). LFOs can originate from neural activity and systemic autoregulation and might have important implications for perivascular fluid clearance though the IPAD and glymphatic systems.3,4 Typically, LFOs are measured using Blood-oxygenation-level-dependent (BOLD) resting-state functional MRI (rs-fMRI)5,6 with several studies have correlating AD progression and declined intrinsic brain activity with disrupted LFOs.7,8 Furthermore, studies that assessed both rs-fMRI and single-delay arterial spin labeling (ASL) have reported disrupted low-frequency fluctuations and decreased cerebral blood flow (CBF) in cognition-related regions in AD subjects. These studies demonstrated higher accuracy in differentiating AD and healthy cohorts9 when combining MRI techniques. However, single-delay ASL is sensitive to prolonged transit time, which can particularly affect aging and diseased groups. Moreover, LFOs measures from BOLD signals are sensitive to motion contamination.10,11 Therefore, in this study we aimed to systematically investigate the effects of patient motion and prolonged transit time during MR scans in AD subjects, and the effects on LFOs and CBF in measurements. To improve image quality of the CBF mapping, perfusion estimates were implemented with transit-time insensitive multi-delay ASL (MD-ASL).Methods
Subjects: A total of 109 subjects were recruited to this study, including subjects with clinically diagnosed Alzheimer’s Disease (AD, n=24), mild cognitive impairment (MCI, n=29), and cognitive normal controls (Control, n=56). Images were collected on 3.0T MRI systems (MR750, GE Healthcare) using a 32-channel head coil.Image acquisition: rs-fMRI was performed with a 2D simultaneous multi-slice EPI sequence (TR=0.8 s, 400 repetitions, isotropic resolution=2 mm). MD-ASL was performed with pseudo-continuous tagging and a 3D multi-shot FSE stack-of-spirals readout (TR/TE=9933/11.64 ms, Hadamard encoded PLD=1.0/1.9/2.9 s, resolution=1.875x1.875x4 mm2). A reference scan was obtained immediately followed by MD-ASL sequence to compensate for coil sensitivity and equilibrium magnetization of blood. A set of high-resolution T1-weighted MPRAGE was also acquired for anatomical reference (TR/TE=5.4/2.4 ms, resolution=0.5x0.5x0.8 mm3).
Image processing: Pre-processing steps including motion correction, segmentation, and registration were performed using SPM12 (Wellcome Trust Centre for Neuroimaging, University of College London, UK). The first 10 rs-fMRI frames were discarded. The remaining time frames were spatially aligned to the 1st frame, where the corresponding transformations were extracted for head motion estimates. Translation/rotation range and SD were calculated over the time course, metrics in three directions were combined with a L2 norm. A 2D Gaussian smoothing kernel (FWHM=6mm) was applied. BOLD signal time course was converted into frequency domain by fast Fourier transform. Spectral power was normalized to its Fourier coefficient at zero frequency. Amplitude of low-frequency fluctuation (ALFF) was obtained by summarizing spectral power within frequency band at 0.01-0.08 Hz. ASL CBF mapping was computed by using a single-compartment model followed by combining results from multiple delays for transit-time correction.12,13The resultant CBF map was transferred to rs-fMRI space by applying a spatial transform generated from registering ASL reference scan to the 1st frame of rs-fMRI by using normalized mutual information.T1-weighted images were segmented into tissue probability maps, and then registered to rs-fMRI space using normalized mutual information. Regions-of-interest (ROI) of gray matter (GM), white matter (WM), cerebrospinal fluid (CSF) were extracted from the registered probability maps (threshold = 80%). A global whole brain (WB) mask was also obtained from a combination of GM and WM probability maps (threshold = 95%). ROI medians for CBF and ALFF values were then extracted from the resulting global masks.
Results
By visual inspection, images from 92 subjects were found to have complete dataset, sufficient signal-to-noise ratio and minimal artifact contamination. Additional criterion was applied to reject head motion exceeding 1 mm during rs-fMRI scan, resulting in 69 subjects remaining (AD n=14, MCI n=14, Control n=41). Summary of demographic information was listed in Table 1. Figure 1 shows translation and rotation motion estimates separated by disease in all 109 subjects, demonstrating significant differences between age matched controls, and subjects with MCI and AD. Taking the motion estimates and plotting with disease (Figure 2), a significant positive correlation is observed between the degree of motion and ALFF measures but not with CBF measures. Figure 3 demonstrates box plots of CBF and ALFF medians extracted from the segmented ROI. Significant differences were found between groups with CBF values extracted from both GM (P < 0.05) and WB masks (P < 0.05). However, no significant difference was found with ALFF measurements. No significant correlation between CBF and ALFF was observed in ROI medians extracted from either GM masks or WB masks (Figure 4).Discussion and conclusions
Based on motion estimation, 23 of total subjects (24%) were excluded from the analysis, where 8 subjects were clinically diagnosed AD, 10 subjects were from MCI cohort, and 5 subjects were cognitively normal controls. Overall, a larger degree of head movement was found in the diseased cohort than the one in the control group. Our results suggested ALFF measurements were less immune to unconscious head movement. Imaging protocol should be carefully designed to minimize potential motion during rs-fMRI scans, especially when implementing on diseased cohorts.Acknowledgements
The authors gratefully acknowledge research support from GE Healthcare and NIH/NIA UF1 AG051216.References
- Iadecola, et al. Neurovascular regulation in the normal brain and in Alzheimer's disease. Review Nat Rev Neurosci. 2004;5:347-60.
- Rivera-Rivera, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer's disease. J Cereb Blood Flow Metab. 2016;36:1718-1730.
- Hawkes, et al. Failure of Perivascular Drainage of β-amyloid in Cerebral Amyloid Angiopathy: Failure of Perivascular Drainage of β-amyloid. Brain Pathology 2014;24:396–403.
- Carare, et al. Vasomotion Drives Periarterial Drainage of Aβ from the Brain. Neuron. 2020;105:400–401.
- Biswal, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-41.
- Zang, et al.Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83-91.
- de Vos, et al. A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer's disease. Neuroimage. 2018;167:62-72.
- Yang, et al. Gradual Disturbances of the Amplitude of Low-Frequency Fluctuations (ALFF) and Fractional ALFF in Alzheimer Spectrum. Front Neurosci. 2018;12:975.
- Zheng, et al. Disrupted Regional Cerebral Blood Flow, Functional Activity and Connectivity in Alzheimer's Disease: A Combined ASL Perfusion and Resting State fMRI Study. Front Neurosci. 2019;13:738.
- Satterthwaite, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623-32.
- Yang, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183-201.
- Alsop, et al. Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magn Reson Med. 2015;73:102–116
- Dai, et al. A Reduced Resolution Transit Delay Prescan for Quantitative Continuous Arterial Spin Labeling Perfusion Imaging. Magn Reson Med. 2012;67:1252–1265.
Figures

Table 1. Summary of demographic information. *: significance level obtained from one-way ANOVA
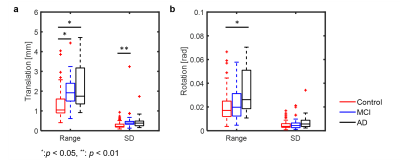
Figure 1. Box plots of motion estimates from the total recruited subjects. Both range and SD of translation estimates were found to be significantly different between groups (P < 0.01), where the control cohort was found to have smaller translational motion than both MCI and AD (P < 0.05). SD calculated from translation metrics also showed significant differences between groups.
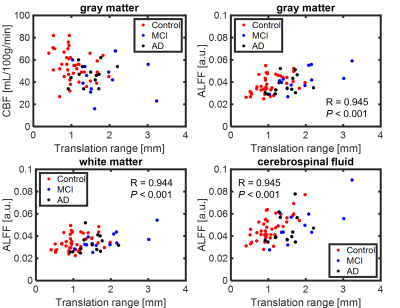
Figure 2. Scatter plots of translational range and quantitative metrics.
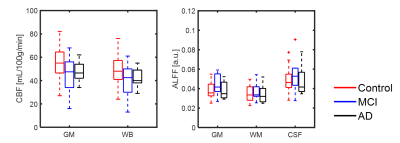
Figure 3. Box plots of CBF and ALFF measurements extracted from gray matter (GM), white matter (WM), CSF, and whole brain masks (WB) global masks. A trend of reduced CBF was observed with AD progression, CBF medians from both GM (P < 0.05) and WB (P < 0.05) were both found to be significantly different between groups. No significant differences were found between groups in ALFF metrics.
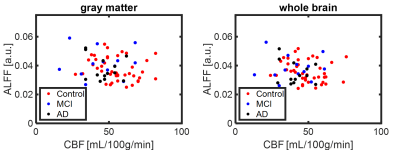
Figure 4. Scatter plot of CBF and ALFF ROI medians. When comparing the results between head motion and quantitative measurements, ALFF results from global masks indicated strong dependency on translational head motion. No significant correlation was found between CBF and translational motion.