1508
Alterations of the sleep-regulating systems in glaucoma1Department of Ophthalmology, New York University Grossman School of Medicine, New York, NY, United States, 2Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
Glaucoma patients have a high incidence of sleep disorders. This relationship implies that glaucoma may involve alterations in sleep-regulating systems. Here, we examined how glaucoma affects sleep-regulating subcortical systems. In particular, we focused on the ventrolateral preoptic nucleus (VLPO), a central sleep-inducing hub. We demonstrated that glaucoma patients had altered functional connectivity of VLPO with the subcortical arousal system and the occipital cortex. We also showed that glaucoma patients had reduced GABA levels in the occipital cortex. Overall, our study suggests that sleep-regulating subcortical structures involving VLPO and their inhibitory projections to the cortex are impaired in glaucoma.
Introduction
Glaucoma patients report a higher prevalence of sleep disorders1,2. Such a connection implies that glaucomatous pathogenesis may involve alterations in sleep-regulating systems. Indeed, increasing evidence indicates that glaucoma involves widespread brain changes3,4. Therefore, we focused on the possible involvements of ventrolateral preoptic nucleus (VLPO), a major sleep-inducing hub in human subcortical structures5 in glaucoma. VLPO has been considered an 'off' switch that induces sleep by sending inhibitory signals to the subcortical arousal systems and the cortex6,7. In this study, we hypothesized that the sleep-regulating subcortical systems involving VLPO and their inhibitory projections to the cortex are impaired in glaucoma. To test this hypothesis, we examined whether VLPO exhibits altered resting-state functional connectivity with the subcortical arousal systems and the cortex. Then, we examined if the neurochemical balance between excitatory and inhibitory neurotransmitters (E/I balance) is altered in the cortical areas where VLPO exhibits weaker connectivity in glaucoma patients.Methods
38 glaucoma patients and 22 healthy control subjects were scanned for resting-state fMRI inside a 3-Tesla Siemens Allegra MRI scanner (Siemens Healthcare, Erlangen, Germany). Additionally, 15 glaucoma patients and 4 healthy control subjects were scanned for proton MRS inside a 3-Tesla Siemens Prisma MRI scanner (Siemens Healthcare, Erlangen, Germany). For anatomical localization, high-resolution T1-weighted MR images were acquired using a multi-echo magnetization-prepared rapid gradient echo sequence (voxel size= 1×1×1 mm3). Functional MR images were acquired using a gradient-echo echo-planar imaging (EPI) sequence covering the whole brain while subjects are at rest with eyes closed (TR/TE=2000/26 ms; voxel size=2x2x3 mm3). MRS for gamma-aminobutyric acid (GABA) was obtained using a MEGA-PRESS prototype sequence (TR/TE= 1500/68 ms) with double-banded pulses. MRS for glutamate was obtained using a PRESS sequence (TR/TE= 3000/30 ms). The same single voxel (2.2×2.2×2.2 cm3) was placed along the calcarine sulci in the most posterior part of the occipital lobe bilaterally for both MEGA-PRESS and PRESS sequences.fMRI data were analyzed using the Functional Connectivity toolbox. Preprocessing was performed using the default pipeline. ROIs were defined in MNI space at the coordinates of VLPO, posterior hypothalamus (PH), dorsal raphe (DR), median raphe (MR), locus coeruleus (LC), and habenula as reported from the previous mapping in the human brains8-11.
Glutamate and GABA were separately fitted by LCModel12. We normalized the amount of GABA and glutamate using NAA values obtained from MEGA-PRESS, following LCModel guidelines12. We calculated the E/I balance by dividing the amount of glutamate by that of GABA13.
Results
At the subcortical level, we examined the functional connectivity between VLPO and components of the arousal systems, including PH, DR, MR, LC, and habenula (Figure 1). We observed that VLPO and PH's functional connectivity was enhanced in glaucoma patients (P=0.048, two-tailed t-test; Figure 2A, B). Specifically, in healthy subjects, VLPO and PH was negatively correlated, suggesting mutual inhibition (Figure 2B). However, VLPO and PH's connectivity shifted in a positive direction in glaucoma patients (Figure 2B). In addition, habenula and MR had stronger positive connectivity in glaucoma patients (P=0.006, two-tailed t-test; Figure 2A, C).At the cortical level, VLPO had reduced functional connectivity with the medial occipital cortex (P=0.014, two-tailed t-test; Figure 3A, B, C) and posterior occipital cortex (P=0.006, two-tailed t-test; Figure 3A, B, D) in glaucoma patients. The functional connectivity between VLPO and medial occipital network or the posterior occipital network was positive in healthy controls. However, these connections became reduced close to zero in glaucoma patients (Figure 3C, D).
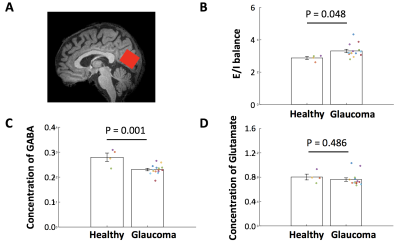
Since we observed decreased functional connectivity of VLPO in the occipital cortex, we measured the amount of GABA, glutamate, and the E/I balance from the occipital cortex (Figure 4A). We observed that the amount of GABA was reduced in the occipital cortex of glaucoma patients (P=0.048, two-tailed t-test; Figure 4C), resulting in enhanced E/I balance (P=0.001, two-tailed t-test; Figure 4B). In contrast, the amount of glutamate was comparable between glaucoma and healthy groups (P=0.486, two-tailed t-test; Figure 4D).
Discussion
Our study demonstrated that the sleep-regulating subcortical systems involving VLPO and their projections to the cortex are impaired in glaucoma. At the subcortical level, VLPO showed enhanced connectivity with PH, one of the arousal systems. Considering that healthy subjects present negative connectivity (mutual inhibition) between VLPO and PH, this result suggests that VLPO may not effectively inhibit the arousal systems in glaucoma.At the cortical level, VLPO presented weaker functional connectivity with the occipital cortex. This finding implies that the degree of inhibition that VLPO exerts to the occipital cortex is impaired in glaucoma. Additionally, the occipital cortex exhibited a reduced GABA amount, making the neurochemical environment an excitatory-dominant state. While the relationship between reduction of functional connectivity and GABA amount remains elusive, it is likely that the decreased GABA may also result in loss of inhibition.
Conclusion
Our study demonstrated that glaucoma involves alterations in the sleep-regulating systems involving VLPO. Such alterations are likely to underlie the high prevalence of sleep disorders in glaucoma. Future studies could address if these alterations are associated with clinical assessments or sleep quality. It may also open a new line of studies testing the feasibility of new treatments, such as non-invasive transcranial ultrasound stimulation of VLPO or oral administration of GABA supplements for glaucoma.Acknowledgements
This work was supported in part by the National Institutes of Health R01-EY028125 and P41-EB017183 (Bethesda, Maryland); BrightFocus Foundation G2013077, G2016030, and G2019103 (Clarksburg, Maryland); Research to Prevent Blindness/Stavros Niarchos Foundation International Research Collaborators Award (New York, New York), and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (New York, New York).References
1. Qiu, M., P.Y. Ramulu, and M.V. Boland, Association Between Sleep Parameters and Glaucoma in the United States Population: National Health and Nutrition Examination Survey.Journal of Glaucoma, 2019;28(2):97-104.
2. Wang, H., et al., Changes in the circadian rhythm in patients with primary glaucoma.PLoS One, 2013;8(4):e62841.
3. Trivedi, V., et al., Widespread brain reorganization perturbs visuomotor coordination in early glaucoma.Sci Rep, 2019;9(1):14168.
4. Faiq, M.A., et al., Cholinergic nervous system and glaucoma: From basic science to clinical applications.Prog Retin Eye Res, 2019;72:100767.
5. Saper, C.B. and P.M. Fuller, Wake-sleep circuitry: an overview.Curr Opin Neurobiol, 2017;44:186-192.
6. Sherin, J.E., et al., Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat.J Neurosci, 1998;18(12):4705-4721.
7. Suntsova, N., et al., Sleep-waking discharge patterns of median preoptic nucleus neurons in rats.J Physiol, 2002;543(Pt 2):665-677.
8. Keren, N.I., et al., In vivo mapping of the human locus coeruleus.Neuroimage, 2009;47(4):1261-1267.
9. Beliveau, V., et al., Functional connectivity of the dorsal and median raphe nuclei at rest.Neuroimage, 2015;116:187-195.
10. Baroncini, M., et al., MRI atlas of the human hypothalamus.Neuroimage, 2012;59(1):168-180.
11. Lawson, R.P., W.C. Drevets, and J.P. Roiser, Defining the habenula in human neuroimaging studies.Neuroimage, 2013;64:722-727.
12. Provencher, S.W., Automatic quantitation of localized in vivo 1H spectra with LCModel.NMR Biomed, 2001;14(4):260-264.
13. Bang, J.W., et al., Consolidation and reconsolidation share behavioural and neurochemical mechanisms.Nature Human Behaviour, 2018;2(7):507-513.
Figures