1507
The changes of regional homogeneity and voxel-mirrored homotopic connectivity in Preschool Children with sensorineural hearing loss: a resting-state fMRI1Department of Imaging, Affiliated Hospital of Guizhou Medical University, Gui yang, China, 2Department of Otorhinolaryngology, Affiliated Hospital of Guizhou Medical University, Gui yang, China, 3GE Healthcare, MR Research, Beijing, China
Synopsis
Long-term hearing loss can lead to changes in brain structure and function in preschool children with sensorineural hearing loss(SNHL). we uses regional homogeneity(ReHo) and voxel mirror homology connectivity(VMHC) methods to evaluate the abnormal changes of functional activities in regional brain and interhemispheric functional connections in children after hearing deprivation during resting-state. The results of this study showed that the structure and function of auditory related brain regions were reorganized, and the interhemispheric information transfer was also changed, which would contribute to a better understanding the neuropathological mechanism of preschool deafness.
Introduction
Congenital sensorineural hearing loss(SNHL) is a type of deafness that occurs prior to language development, approximately 1‰ to 6‰ newborns suffering from severe to profound sensorineural hearing loss[1]. Early hearing deprivation could affect not only language, but cognitive functions[2]. The development of auditory pathway is abnormal due to the long-term lack of sound stimulation in children, which eventually leads to the changes and recombination of auditory-related brain area structure and function[3]. Some prevenient Neuroimaging studies have contributed to our understanding of brain alterations and neuroplasticity in children with hearing loss[4]. however, the reorganization of the visual and language central cortex of SNHL in preschool children and their relationship remain unclear. This study uses regional homogeneity (ReHo) and voxel mirror homology connectivity (VMHC) methods to explore the abnormal changes in regional brain functional activities in children with SNHL, the functional connections between symmetrical alleles in the bilateral cerebral hemispheres, and synchronization of neuroactivity. It provides a reliable basis for understanding the neuropathological mechanism of SNHL cortical reorganization, thereby helping to predict speech and language rehabilitation after CI.Material and Methods
The study was approved by the local Institutional Review Board and all the informed written consents were obtained from all parents of participants. A total of 42 preschoolers with SNHL(20 males and 22 females, mean age 3.29±1.46years) and 32 age-matched healthy controls(17 males and 15 females, mean age 3.09±1.78years) were enrolled. All participants underwent MR scanning on a 3.0 T MRI scanner (Discovery MR 750W, GE Healthcare, Waukesha, USA) including a 3D_T1WI acquisition and blood oxygen level dependent functional MRI(BOLD-fMRI). Images were obtained axially using a gradient echo-planar imaging(EPI) sequence, parameters were as follows: repetition time(TR) = 2000 ms, echo time(TE) = 30 ms, section thickness = 3.4 mm, gap = 3.4 mm, field of view(FOV) = 220 mm × 220 mm, acquisition matrix = 64 × 64, and flip angle(FA) = 90°, 35 slices, volume = 7000. High-resolution structural T1-weighted scan (Three-dimensional Brain Volume, 3D BRAVO) were performed with the following parameters: TR/TE = 9.2/ 4.6 ms, section thickness = 1.6 mm, gap = 0.8 mm, FA = 8°, FOV = 220 mm × 220 mm, and acquisition matrix = 64 × 64, sections = 180. The acquisition data were preprocessed and analyzed by ReHo and VMHC using DPASF 4.3 software package on Matlab 2013b platform. Two-sample t-test was used to compare the difference of regional brain function between SNHL patients and HC, and the synergetic changes between bilateral cerebral hemispheric allelic voxels(P<0.05). Gender and head movement were selected as covariates. Each of SNHL and HC groups were tested with auditory brainstem response(ABR), the ABR of SNHL group was(99.97±7.08)dB nHL, and the ABR of HC group was(15.21±4.66)dB nHL.Rsults
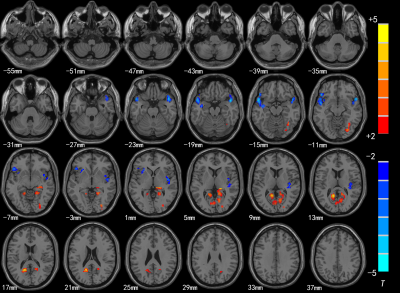
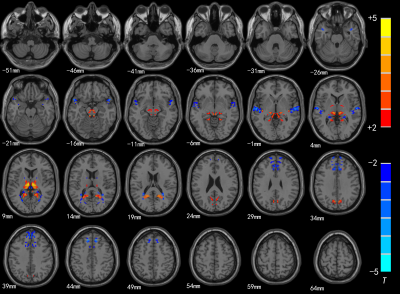
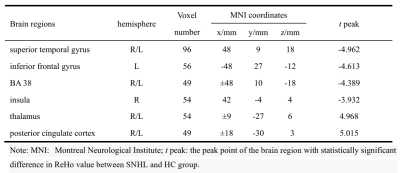
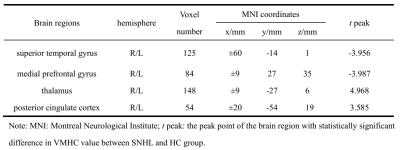
Compared with HC group, the brain areas with decreased ReHo value in the SNHL group including the bilateral superior temporal gyrus(STG), insula(R) and inferior frontal gyrus(L), and significantly increased in the posterior cingulate cortex(PCC) thalamus and bilateral BA 38(Fig.1 and Table 1); in the SNHL group the decreased VMHC value was found in the the bilateral medial prefrontal gyrus and STG, while significantly increased VMHC intensity was found in the bilateral thalamus and posterior cingulate cortex(Fig.2 and Table 2).Discussion and Conclusion
The results suggested that the function of auditory language-related and cognitive brain cortex in SNHL group was affected. ReHo reflects the similarities or synchronization of functional activities in local brain areas, and VMHC offers a metric to evaluate interhemispheric functional connection, which measures integrity of information communication between brain hemispheres[5,6]. In the study, decreased ReHo could reflect the desynchronized blood flow which indicated the activity of the auditory cortex was desynchronized in SNHL group, while reduced VMHC may be associated with decreased interhemispheric information transfer and coordination; increased ReHo and VMHC may be functional compensation or cross-mode recombination of auditory-related brain area after long-term hearing deprivation. So, ReHo and VMHC can be used to analyze the changes of brain network function in preschool children with SNHL in a more comprehensive way. At the same time, it provides the underlying pathological mechanisms of preschool children with SNHL.Acknowledgements
No acknowledgement found.References
[1] Xia S, et al. Neural Plast, 2017,2017: 1-8.
[2] Hall ML, et al. J Speech Lang Hear Res. 2018;61(8):1970-1988.
[3] Wang S, et al. Dev Cogn Neurosci, 2019,38:1-9.
[4] Wang, SS et al. Developmental cognitive neuroscience vol. 38 (2019):100654.
[5] Zhao J, et al. Front Aging Neurosci. 2020;12:20. Published 2020 Feb 26.
[6] Paakki JJ, et al. Brain Res. 2010;1321:169-179.
Figures