1506
Aberrant spontaneous low-frequency brain activity in patients with subjective cognitive decline: A resting-state fMRI study1Radiology department, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2Rehabilitation Department, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 3MR Research China, GE Healthcare, Beijing, China
Synopsis
In this study, whole brain amplitude of low-frequency fluctuation (ALFF) changes have been respectively investigated for patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI) and healthy controls (HCs). Relative to HCs, significantly lower ALFF values have been separately found in the regions of right supramarginal gyrus, left precuneus and right supplementary motor area for SCD and MCI patients. Additionally, the ALFFs at these regions also showed strong correlations with multiple clinical scales. Therefore, ALFF might be considered an effective index in the early detection of SCD patients.
Introduction
With the increased understanding of Alzheimer diseases’ (AD) etiology, the National Institute on Aging and Alzheimer’s Association (NIA-AA) suggested that AD should be sub-divided into AD-preclinical stage, AD-mild cognitive impairment (MCI) stage and AD-dementia stage. The preclinical phase of AD contains neuronal degeneration and amyloid deposition with subtle cognitive decline (SCD stage)1. Early diagnosis of SCD and MCI has become the key to prevent from dementia. So far, many MRI studies have found reduced amplitude of low-frequency fluctuation (ALFF) in AD patients2,3 . A meta-analysis of resting-state (rs)-fMRI studies have indicated that, compared to healthy controls (HC)s, MCI patients showed decreased ALFFs in the bilateral precuneus/posterior cingulate cortices, bilateral frontal-insular cortices, left occipitotemporal cortex and right supramarginal gyrus4. However, changes in ALFF and the correlations between ALFF and clinical cognitive evaluations in SCD patients remain unknown.To investigate this, ALFF was systematically evaluated in patients with SCD and MCI as well as HCs for comparison. Additionally, the correlations between the ALFF values of all subjects and multiple clinical scales were estimated respectively.
Materials and Methods
Subjects26 clinically confirmed SCD patients (mean age: 71.04±6.96 years) and 25 (mean age: 71.42±5.90 years) MCI patients were recruited in this study. 27 HCs (mean age: 71.48±6.88 years) were also included for comparison. Each subject was assessed with multiple clinical scales, including Mini-Mental-State-Examination (MMSE), Montreal-Cognitive-Assessment (MoCA), Wechsler-Memory-Scale-Revised-logical-memory-Test, Trail-Making-Test (TMT) A&B, Auditory-Verbal-Learning-Test (AVLT), Boston-Naming-Test, Functional-Activities-Questionnaire, Short-Form-Health-Survey and Geriatric-Depression-Scale.
MRI experiment
All MR experiments were performed for each subject at a 3T-MR scanner (Discovery 750W, GE Healthcare, USA) equipped with a 24-channel head coil after a written consent was obtained. A fast-spoiled- gradient-echo based 3D-BRAVO sequence was employed to acquire 1mm³-isotropic T1-weighted (T1w) MR images. The corresponding scan parameters were of field- of-view (FOV)= 256x256mm², repetition time (TR)=8.5ms, echo time (TE)=3.2ms, inversion time (TI)=450ms, flip angle (FA)=12degree, number of slices=188, slice thickness=1mm, matrix size=256x256 and bandwidth=31.25kHz. In rs-fMRI experiment, a multi-phase single-shot echo-planar-imaging sequence was applied for blood-oxygen-level-dependent (BOLD) imaging acquisition. The scan parameters applied were shown as follows: FOV=224X224mm², TR=2000ms, TE=30ms, FA=90degree, matrix size=64x64, number of slices=33, slice thickness=3.5mm, slice gap=0.7mm and number of phases=240. Total scan time was less than 13 minutes.
Data analysis
All pre-processing procedures were performed using the Data Processing & Analysis for Brain Imaging (DPABI_V5.0 http://rfmri.org/dpabi). After preprocessing, the ALFF of each voxel was defined by averaged square root of the power spectrum of time series, computed in a frequency domain based on a fast Fourier transformation and averaged across the frequency interval of 0.01–0.08 Hz. The statistical analysis of standardized ALFF data was carried out by the statistical module of DPABI. One-way analysis-of-variance (ANOVA) with the factor of group and subsequent post-hoc-t tests were applied to detect the difference of ALFF among SCD, MCI patients and HCs (GRF correction, voxel p value<0.001, cluster p value<0.05). In addition, Pearson correlation analysis was separately employed to evaluate the relationship between ALFFs of all subjects and each of clinical scale scores in SPSS software 23.0. Significance threshold was set as p<0.05.
Results
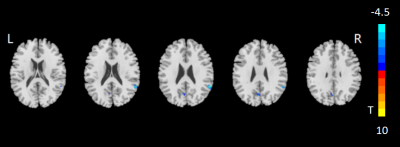
ANOVA analysis revealed significant different ALFF among patients with MCI and SCD and HC groups in the right supramarginal gyrus, left precuneus and right supplementary motor area. The corresponding post-hoc-t test further indicated that compared with HC group, both MCI and SCD groups showed lower ALFF values at the right supramarginal gyrus and left precuneus region (Figs.1,2; GRF correction, voxel p value<0.001, cluster p value<0.05). Lower ALFF values at right supplementary motor area were also found in SCD group than HC group.Using Pearson correlation analysis (Tables 1,2,3), the MoCA was positively correlated with the ALFF values of right supramarginal gyrus (r=0.349, p=0. 002) and left precuneus (r=0.373, p=0.001) in all subjects. The MMSE was positively correlated with the ALFF values of left precuneus (r=0.278, p=0.0.014) and right supplementary motor area (r=0.232, p=0.041). Meanwhile, a significant positive correlation was revealed between the ALFF values at left precuneus (r=0.363, p=0.001) and Boston-Naming-Test. Additionally, the ALFF values of right supramarginal gyrus (r=-0.258, p=0.023) and left precuneus (r=-0.340, p=0.002) showed a significant negative correlation with TMT-B score, respectively.
Discussion and conclusion
In this study, we systematically investigated the ALFF values among SCD and MCI patients and HCs. Significantly lower ALFF values for the right supramarginal gyrus, left precuneus, and right supplementary motor area regions were shown in SCD patients than HCs, indicating that early changes of ALFF might be a biomarker in SCD individuals. These regions are related to language perception and processing, episodic memory and motor actions and bimanual control.Additionally, the ALFF values of the right supramarginal gyrus, left precuneus and right supplementary motor area were strongly correlated with multiple clinical scales. We thus infer that for SCD and MCI patients, reduced ALFF in right supramarginal gyrus and left precuneus might result in the decrease of MMSE, MoCA and Boston-Naming-Test scores, as supramarginal gyrus is involved in language perception and processing, and the precuneus is involved in episodic memory. In conclusion, ALFF might be considered an effective biomarker in the early detection of SCD patients.
Acknowledgements
No acknowledgement found.References
1.Jack CR, Jr., Bennett DA, Blennow K et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535-62.
2.Liu X, Wang S, Zhan X et al. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer's disease. J Alzheimers Dis. 2014;40:387-97.
3.Wang Z, Yan C, Zhao C et al. Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer's disease: a resting-state functional MRI study. Hum Brain Mapp. 2011;32:1720-40.
4.Pan P, Zhu L, Yu T et al. Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: A meta-analysis of resting-state fMRI studies. Ageing Res Rev. 2017;35:12-21.
Figures