1505
Pathological changes in subcortex disrupt cortical synchronization and metastability affecting cognitive function in Parkinson’s disease1Zhejiang University, Hangzhou, China
Synopsis
In this paper, we investigated spatiotemporal dynamics of the phase interactions among resting-state blood oxygen level-dependent signals of Parkinson’s disease patients (n = 159) and normal controls (n = 152). We demonstrated that diminished dopaminergic function and the pathological changes in thalamus related structures responsible for decreased cortical synchronization and metastability, further affect cognitive function in Parkinson’s disease.
Abstract
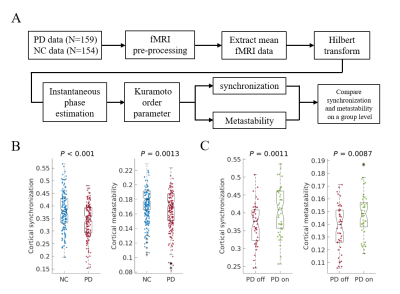
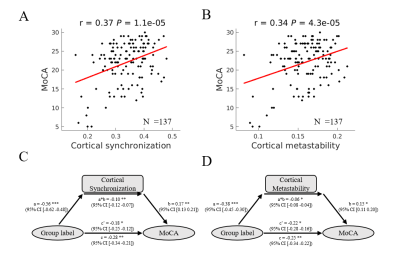
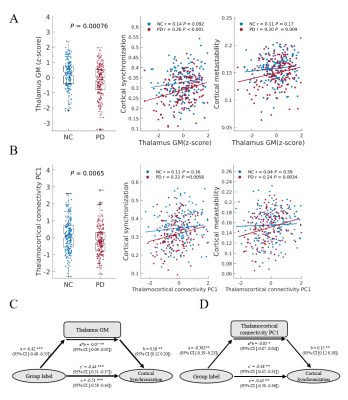
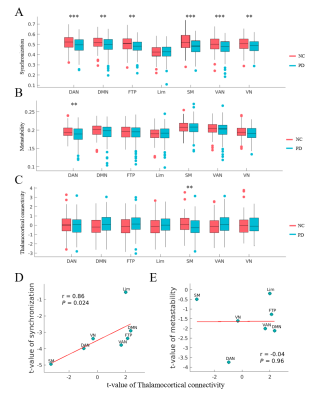
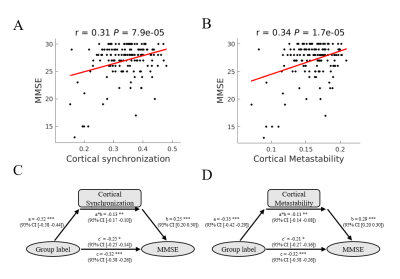
Parkinson’s disease is primarily characterized by loss of dopaminergic cells and atrophy in subcortical regions. However, the impact of these changes on large-scale dynamic integration and segregation of cortex are not well understood. In this paper, we investigated spatiotemporal dynamics of the phase interactions among resting-state blood oxygen level-dependent signals of Parkinson’s disease patients (n = 159) and normal controls (n = 152). We found cortical synchronization and metastability in Parkinson’s disease patients are significantly decreased. To examine the causal role of dopamine depletion in cortical synchronization and metastability, we investigated 45 Parkinson’s disease patients, both at OFF (at least 12 hours after withholding dopaminergic drugs) and ON (one hour after administration of 200 mg L-dopa and 50 mg benserazide) state. We found that cortical synchronization and metastability in Parkinson’s disease patients are significantly increased in the ON state. Furthermore, the extent of cortical synchronization and metastability in the OFF state reflected cognitive performance and mediate the difference of cognitive performance between Parkinson’s disease patients and normal controls. In addition, we demonstrated that measures of both the thalamus total grey matter volume and subcortical-cortical structural connectivity had a positive relationship with cortical synchronization and metastability in the dopaminergic OFF state. No such relationships were found in normal controls. Mediation analysis showed that the difference of thalamus total grey matter volume and thalamocortical structural connectivity mediate the difference of cortical synchronization between Parkinson’s disease patients and normal controls. Finally, we found decreases in synchronization of each cortical networks is correlated to impairment in its structural connectivity to thalamus and caudate. Together, these results highlight diminished dopaminergic function and the pathological changes in thalamus related structures responsible for decreased cortical synchronization and metastability, further affect cognitive function in Parkinson’s disease.Acknowledgements
We thank all patients with Parkinson’s disease patients and healthy controls who participated in this study.References
Aarsland, D., Creese, B., Politis, M., Chaudhuri, K. R., Ffytche, D. H., Weintraub, D., & Ballard, C. (2017). Cognitive decline in Parkinson disease. Nat Rev Neurol, 13(4), 217-231. doi:10.1038/nrneurol.2017.27
Alderson, T. H., Bokde, A. L. W., Kelso, J. A. S., Maguire, L., & Coyle, D. (2018). Metastable neural dynamics in Alzheimer's disease are disrupted by lesions to the structural connectome. NeuroImage, 183, 438-455. doi:10.1016/j.neuroimage.2018.08.033
Alderson, T. H., Bokde, A. L. W., Kelso, J. A. S., Maguire, L., & Coyle, D. (2018). Metastable neural dynamics in Alzheimer's disease are disrupted by lesions to the structural connectome. NeuroImage, 183, 438-455. doi:10.1016/j.neuroimage.2018.08.033
Oh, S. W., Harris, J. A., Ng, L., Winslow, B., Cain, N., Mihalas, S., . . . Zeng, H. (2014). A mesoscale connectome of the mouse brain. Nature, 508(7495), 207-214. doi:10.1038/nature13186
Shine, J. M., Bell, P. T., Matar, E., Poldrack, R. A., Lewis, S. J. G., Halliday, G. M., & O'Callaghan, C. (2019). Dopamine depletion alters macroscopic network dynamics in Parkinson's disease. Brain, 142(4), 1024-1034. doi:10.1093/brain/awz034
Figures