1502
Volumetric and connectivity profile of regional thalamic abnormality in amyotrophic lateral sclerosis
Sicong Tu1, Marion Sourty2, Fernando Calamante1, Manojkumar Saranathan3, Ricarda Menke4, Kevin Talbot4, Matthew Kiernan1, and Martin Turner4
1The University of Sydney, Sydney, Australia, 2Université de Strasbourg, Strasbourg, France, 3University of Arizona, Tucson, AZ, United States, 4University of Oxford, Oxford, United Kingdom
1The University of Sydney, Sydney, Australia, 2Université de Strasbourg, Strasbourg, France, 3University of Arizona, Tucson, AZ, United States, 4University of Oxford, Oxford, United Kingdom
Synopsis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative disease with widespread extra-motor cortical and subcortical abnormality. The current findings highlight significant regional volumetric and connectivity abnormality in the thalamus associated with clinical features and may be a promising marker of disease burden.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease of the motor system, with a clinical, pathological, and genetic overlap with frontotemporal dementia1. Disease burden in ALS is not limited to neural structures underlying motor function but shows a diffuse progressive pattern of cortical and subcortical involvement2. The thalamus is a core relay structure projecting to virtually all cortical brain regions. We have previously highlighted that thalamic abnormality is a robust MR disease signature in ALS and demonstrated changes in diffusivity associated with thalamic sub-regions mediating motor and frontal projections3. The integrity of thalamic nuclei and their association with clinical features, however, remains unclear but may be a holistic marker of widespread cortical dysfunction. We present findings from a clinically well-defined cohort of sporadic ALS patients, employing a novel multi-atlas segmentation technique for thalamic nuclei4 and track-weighted functional connectivity5 to characterize volumetric and connectivity profiles of thalamic abnormality.Methods
Forty sporadic ALS patients and 27 age-and-education matched healthy control participants (p values > 0.07) were recruited from the Oxford MND clinic, in accordance with ethical approval. All patients underwent comprehensive clinical examination by an experienced neurologist and MRI scan (3T Siemens Trio; 12 Channel Head Coil) on the same day. T1: MPRAGE, TR=2040ms, TE=4.7ms, flip angle=8°, 1mm isotropic; DWI: 60 directions, b-value=1000s/mm2, TR=10000ms, TE=94ms, 2mm isotropic, 5 x b0 interleaved; rs-fMRI: TR=3000ms, TE=28ms, 3mm isotropic, eyes closed. Thalamic sub-nuclei were robustly segmented on each participant’s T1 image using a variant of the recently proposed Thalamus Optimized Multi-Atlas Segmentation (THOMAS) pipeline4 to accommodate conventional MPRAGE contrast as opposed to white matter nulled MPRAGE (WMn-MPRAGE) used in the original THOMAS implementation. An atlas comprising WMn-MPRAGE data from 20 individuals with 11 manually delineated thalamic nuclei was non-linearly (diffeomorphic) warped from atlas space to native T1 input space using ANTs. The 20 set of thalamic nuclei labels were fused using a majority voting scheme to generate one complete set of segmented nuclei separately for the left and right thalamus (Fig.1). Segmentations were visually inspected for accuracy. Whole-brain track-weighted static functional connectivity (TW-sFC) maps were calculated following previously published guidelines5. Diffusion MRI data were pre-processed using MRtrix and whole-brain probabilistic fibre-tracking was performed using the ACT framework6. Resting-state fMRI data pre-processing included: geometric distortion correction, motion correction and realigned to the distortion corrected mean b0 diffusion image. TW-sFC maps were generated for each participant in MRtrix whereby each voxel represents the mean correlation between BOLD timeseries at the endpoints of each crossing streamline (Fig. 2).Results
Reduced thalamus volume was observed bilaterally in ALS compared to control (p values < 0.04). Thalamic nuclei were first grouped into 4 primary thalamic regions (anterior, medial, ventral, posterior). Bilateral volumetric reduction was consistently observed across all regions except for the anterior thalamus in ALS (Fig. 3; p values < 0.05). For individual thalamic nuclei, only the left mediodorsal and centromedian nuclei demonstrated a significant reduction in ALS compared to controls in our cohort (p values < 0.03). Significant increased TW-sFC was observed in ALS, compared to controls, in the right anterior thalamus (Fig. 4; p =0.03) and right anterior ventral nuclei (p < 0.01); left anterior thalamus approached significance (p = 0.06). TW-sFC of the mediodorsal nuclei showed a consistent correlation with disease duration (left: r = -0.54, p < 0.01; right: r = -0.41, p = 0.02) and disease progression rate (left: r = 0.49, p < 0.01; right: r = 0.41, p = 0.03).Discussion & Conclusions
Regional thalamic abnormalities are present in sporadic ALS patients and hold a significant association with key clinical features. While reduced volume was detected throughout thalamus sub-regions, this was not found to reflect clinical features of disease. In contrast, thalamic connectivity showed a higher degree of variability across the ALS cohort, but demonstrated significant clinical associations with disease duration and progression rate in thalamic nuclei mediating frontal and motor cortical signalling. The findings reinforce that diffusion and functional MR imaging modalities are promising markers for capturing neuropathological disease burden in ALS.Acknowledgements
The authors thank all study participants for their efforts and enthusiasm.References
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942-955. 2. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20-38. 3. Tu S, Menke RAL, Talbot K, Kiernan MC, Turner MR. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018. 4. Su JH, Thomas FT, Kasoff WS, et al. Thalamus Optimized Multi Atlas Segmentation (THOMAS): fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage 2019;194:272-282. 5. Calamante F, Smith RE, Liang X, Zalesky A, Connelly A. Track-weighted dynamic functional connectivity (TW-dFC): a new method to study time-resolved functional connectivity. Brain Struct Funct 2017;222:3761-3774. 6. Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 2012;62:1924-1938.Figures
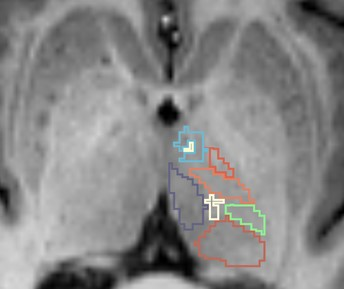
Figure 1. Representative thalamic nuclei segmentation using the
THOMAS pipeline on an individual T1 image.
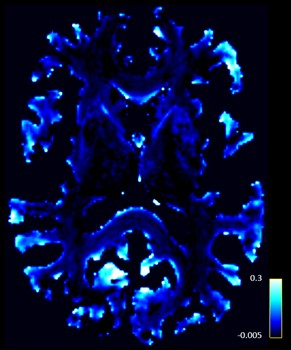
Figure 2. Representative
track-weighted static functional connectivity map for an individual
participant.
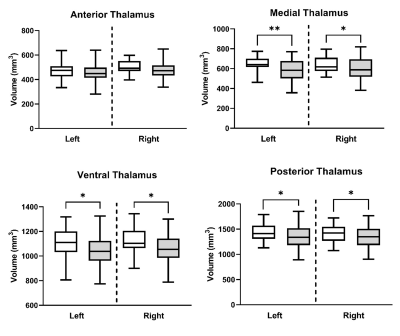
Figure 3. Regional thalamic volumetric reductions in ALS (grey)
compared to control (white). Volumes are normalized for intra-cranial volume
and age. **Indicates significance at p < 0.01. * Indicates significance at p
< 0.05.
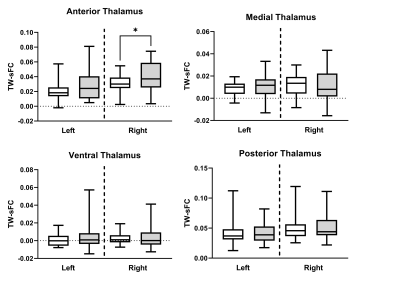
Figure 4. Regional thalamic track-weighted static functional
connectivity (TW-sFC) differences between ALS (grey) and control (white). * Indicates
significance at p < 0.05.