1498
Aberrant Cerebellar Functional Connectivity and its Association with Motor and Non-motor Functions in de novo Drug Naïve Parkinson’s Disease1Center for Advanced Imaging Research, University of Maryland Baltimore, Baltimore, MD, United States, 2Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland Baltimore, Baltimore, MD, United States, 3Department of Neurology, University of Maryland Baltimore, Baltimore, MD, United States
Synopsis
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders characterized by progressive deterioration of motor function as well as a non-motor symptom complex. Resting-state fMRI (rs-fMRI) provides important insights on the pathophysiological mechanisms of PD. Here we used rs-fMRI to systematically investigate the cerebellar functional connectivity changes in de novo drug naïve PD patients compared with healthy controls, and the association between altered cerebellar connectivity and neuropsychological assessments. Our findings support that cerebellar connectivity changes while reflective of early symptoms of PD, also may suggest a possible compensatory mechanism prior to clinical presentation of non-motor features of the disease.
Purpose
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders characterized by progressive deterioration of motor function as well as a non-motor symptom complex [1, 2]. Resting-state fMRI (rs-fMRI) provides important insights on the pathophysiological mechanisms of PD [3, 4]. There is recent evidence from anatomical, clinical, pathophysiological and neuroimaging findings to suggest that the cerebellum may be engaged in cognitive and affective functions beyond motor function [5, 6]. However, most rs-fMRI studies to date focused on the striato-thalamo-cortical pathways or motor function of cerebellum and were performed on patients at relatively advanced stage when patients are already on antiparkinsonian medication. In this study, we used rs-fMRI to systematically investigate the cerebellar functional connectivity (FC) changes in de novo drug naïve PD patients compared with age-matched healthy controls (HC), and to further examine the association between altered cerebellar FC and neuropsychological assessments.Materials and Methods
Data from a cohort of 33 de novo PD patients (20 males/13 females; age of 59.12±10.71 years) was extracted from the Parkinson’s Progression Markers Initiative (PPMI) archive. The resting-state fMRI data and assessments of UPDRS Part III (motor section), Hopkins Verbal Learning Test (HVLT), and Benton’s Judgment of Line Orientation (BJLO) were retrieved at the first available visit before the initiation of antiparkinsonian medication.The rs-fMRI data was acquired on Siemens 3T scanners using a T2* weighted EPI sequence with TR/TE 2400/25 ms; resolution 3.294x3.294x3.3 mm3. Twenty-two age-matched HC was also extracted (17 males/5 females, age of 60.99±10.88 years). The rs-fMRI data were processed using CONN toolbox (ver18) Standard preprocessing pipeline were applied with spatial smoothing (6mm FWHM), temporally band-pass filtering (0.008-0.1 Hz) and nuisance regression of motion parameters and the averaged BOLD signals from WM/CSF to remove non-neuronal artifacts. The Cerebellum was divided functionally into motor cerebellum (Lobule III, IV/V, VI, VIIb, VIII, Vermis I/II, III, IV/V, VI, and VIII) and non-motor cerebellum (Crus I, Crus II, Lobule VIIB, IX, X, vermis VII, IX, and X) as seeds.
The voxel-to-whole brain analysis was performed to estimate the FC map (Pearson correlation). The GLM with covariates of age, gender, and education was applied to estimate FC difference between PD patients and HCs with significance of voxel-level p < 0.005 and cluster-wised FDR p < 0.05 for multiple comparison. For PD group, partial correlation controlled for age, gender, and education was utilized to estimate the association between the FC of significant clusters and neuropsychological assessments with significance of p < 0.05.
Results
Table 1 shows that there is no significant difference in age, gender, education, handedness, and MoCA score between PD patients and HCs. About 97% PD patients were at Hoehn-Yahr stage under 3, 72% of PD patients had less than 6 months PD duration and 67% were predominantly right-side affected.Figure 1 shows that PD patients had decreased FC with all motor cerebellum seeds (except for lobule VIII and vermis VIII) compared with HCs. Most of the decreased FC clusters were located within cerebellum, brain stem, and posterior medial temporal-occipital regions, such as hippocampus, parahippocampal, fusiform cortex (pTFusC), and inferior temporal gyrus (toITG).
Figure 2 shows that PD patients had both increased and decreased FC of the non-motor cerebellum seeds (left/right Crus I, left Crus II, right lobule X, vermis VII and IX) compared with HCs. Increased FC clusters encompassed frontal cortex, sensorimotor cortex, temporal-occipital-parietal associated area, while decreased FC clusters located at caudate and cerebellum. No significant FC changes were observed for other non-motor cerebellum seeds.
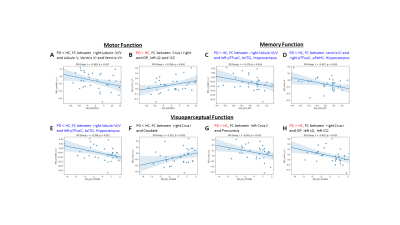
Figure 3A and 3B show that decreased FC between right lobule IV/V to lobule V, Vermis VI and VII and the increased FC between right Crus I and left occipital pole (OP), lingual gyrus (LG) and intracranial cortex (ICC) were associated with worse performance on motor function. Figure 3C, 3D, and 3E show that decreased FC in left or right pTFusC, toITG, and hippocampus were associated better performance of memory and visuoperceptual functions. Figure 3F, 3G and 3H show that decreased FC in caudate and increased FC in precuneus and OP, left LG and ICC were associated with worse performance of visuoperceptual function.
Discussion and Conclusion
Discussion and Conclusion: This study used lobule and vermis-based rs-fMRI FC analysis to investigate the role of the cerebellum in de novo drug naïve PD patients. The PD patients performed worse on motor function which was associated with decreased FC within the cerebellum while also associated with increased FC between Crus I to occipital pole, lingual and intracranial cortex. Decreased FC within hippocampus, fusiform, and inferior temporal gyrus was associated with worse performance on memory function for PD patients, despite comparable performance on verbal memory between PD and HC subjects. Decreased FC between Crus I and caudate was associated with worse performance in visuoperceptual function but conversely increased FC between non-motor lobule region (Crus I and Crus II) with precuneus, lingual and intracranial cortex. These findings suggest multimodal disruption of functional connectivity including those related to motor and cognition within the cerebellum and various brain regions in de novo drug naïve PD patients. These changes while reflective of early symptoms of PD, also suggest a possible compensatory mechanism prior to clinical presentation of non-motor features of the disease.Acknowledgements
Acknowledgement: This work was supported in part by the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS098249 and by the Michael J. Fox Foundation (MJFF) which provides the data source.References
1. Bjornevik, K., M.A. Schwarzschild, and A. Ascherio, Big health data and Parkinson's disease epidemiology: Challenges and opportunities. Parkinsonism Relat Disord, 2020. 71: p. 58-59.
2. Marras, C., et al., Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis, 2018. 4: p. 21.
3. Tessitore, A., M. Cirillo, and R. De Micco, Functional Connectivity Signatures of Parkinson's Disease. J Parkinsons Dis, 2019. 9(4): p. 637-652.
4. Tuite, P., Brain Magnetic Resonance Imaging (MRI) as a Potential Biomarker for Parkinson's Disease (PD). Brain Sci, 2017. 7(6).
5. Lewis, M.M., et al., The role of the cerebellum in the pathophysiology of Parkinson's disease. Can J Neurol Sci, 2013. 40(3): p. 299-306.
6. Mirdamadi, J.L., Cerebellar role in Parkinson's disease. J Neurophysiol, 2016. 116(3): p. 917-9.
Figures
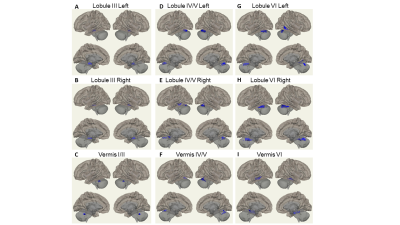
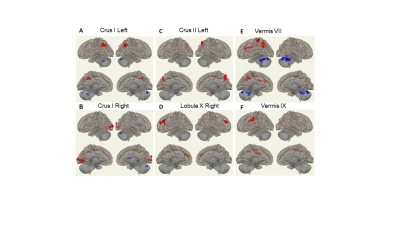
Figure 2: FC difference map between the PD patients and HCs of non-motor cerebellum seeds of (A) left Crus I; (B) right Crus I; © left Crus II; (D) right Lobule X; (E) vermis VII; (F) Vermis IX. Two-sided two sample t-test was performed with significant level of voxel uncorrected p < 0.005 and cluster FDR-corrected p < 0.05. Blue color represents FC of PD less than FC of HCs. Red color represents FC of PD greater than FC of HCs.