1497
Meta-analytic investigation and funtional decoding on neural correlates of high familial risk for mood disorders1West China Hospital of Sichuan University, Chengdu, China
Synopsis
Despite the absence of positive clinical manifestations, there may be neurofunctional signatures of genetic vulnerability in the relatives of patients with mood disorders. We performed a meta-analysis integrating task-based fMRI studies comparing unaffected relatives of patients with mood disorders and healthy controls. Hyper-activation in the insula, as well as hypo-activation in the inferior parietal cortex and precuneus were identified in the high-risk relatives. Functional decoding further suggested emotion-related dysfunction might be primarily associated with abnormal activation pattern among deficits in multiple behavioral domains. These neurofunctional correlates may serve as high-risk biomarkers underlying illness onset and development in mood disorders.
Introduction
Mood disorders, such as major depressive disorder (MDD) and bipolar disorder (BD) are highly prevalent, persistent and impairing diseases primarily, characterized by the disturbance of affective state1. Cumulative epidemiological evidence has supported that MDD and BD are prone to run in families, with heritability estimated to be 37% and 75% in the first-degree relatives2,3. Despite the absence of positive clinical manifestations, neuroimaging biomarkers of genetic vulnerability have been identified in high-risk relatives4,5. Given the overlapping signature across clinical, genetic and neuroimaging aspects in high-risk relatives, investigation towards the non-symptomatic unaffected individuals at high familial risk for mood disorders may therefore provide an essential insight into shared neurobiological underpinnings of illness onset and development.The issue of reliability and reproducibility in individual neuroimaging studies makes meta-analytic approaches promising to provide solutions identifying robust findings by conjunction analysis of original studies6. Of note, transdiagnostic meta-analyses further enabled researchers to explore shared neural substrates of similar disorders robustly, extending our understandings from traditional diagnostic boundaries towards novel endophenotypes7,8. Herein, we conducted a meta-analysis of functional magnetic resonance imaging (fMRI) studies to identify a transdiagnostic task-based activation pattern in unaffected relatives of patients with mood disorders.
Methods
We performed an exhaustive search to identify eligible task-based fMRI studies comparing unaffected relatives at risk for MDD/BD with healthy controls (HC). Seed-based d Mapping with Permutation of Subject Images (SDM-PSI) toolbox was used to perform the following whole-brain voxel-wise meta-analyses9. Significant clusters were identified under the threshold of family-wise error (FWE) corrected p < 0.05.We firstly pooled all the included experiments to identify shared functional abnormalities in the relatives of patients with mood disorders. To obtain more objective and quantitative inferences for the experiment-general findings, we decoded the abnormal functional activation pattern in high-risk relatives via Neurosynth platform10. We calculated Pearson correlation coefficients (CC) between the identified activation map in high-risk relatives and term-based activation maps embedded in Neurosynth. We reported top terms with the highest CC for decoding, as well as the CC of five classical behavioral domain for decomposition.
We further conducted disorder-specific, experiment-specific and age-specific subgroup analyses to characterize the specificity of main findings. Meta-regression was performed to explore the relationship between abnormal functional activation and sex ratio.
Results
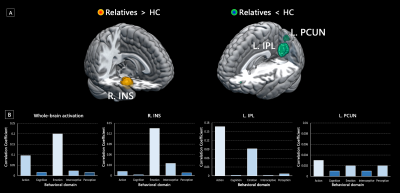
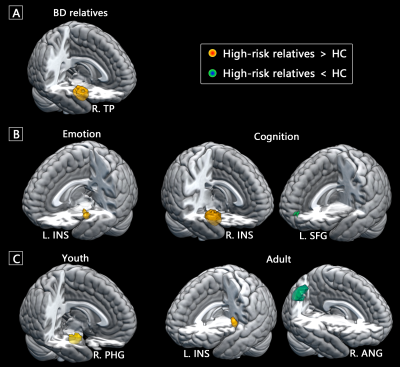
A total of 39 fMRI studies with 44 experiments comparing 1121 relatives of patients with mood disorders (mean age, 27.4 years) and 1367 HC (mean age, 27.3 years) were included in the current meta-analysis. Compared with HC, the relatives of patients with mood disorders exhibited hyper-activation in the right insula extending to surrounding limbic regions, as well as hypo-activation in the left inferior parietal lobule (IPL) and precuneus (Figure 1A). In the functional decoding analysis of whole-brain activation pattern in high-risk relatives, we identified primary association with emotion-related terms and secondary association with cognitive and perceptive terms. Behavioral decomposition consistently found emotion predominantly accounted for the whole-brain functional abnormalities as well as insular abnormalities, and action dominated the parietal abnormalities (Figure 1B).The relatives of BD patients exhibited significant hyper-activation in the limbic regions, while no significant clusters with abnormal activation were identified in the relatives of MDD patients (Figure 2A). We found hyper-activation in the left insula when pooling the emotion-related experiments. Hyper-activation in the right insula and hypo-activation in the left medial superior frontal cortex were identified for the cognition-related experiments (Figure 2B). Adult relatives showed hyper-activation in the left insula as well as hypo-activation in the right angular gyrus, while young relatives showed hyper-activation in the right parahippocampal gyrus (Figure 2C). Meta-regression failed to identify any significant correlation between functional hypo- or hyper-activation and sex ratio in the included studies.
Discussion
To our knowledge, this is the first transdiagnostic quantitative meta-analysis investigating neurofunctional correlates of genetic vulnerability in mood disorders. We combined the relatives of both MDD and BD patients, which could not only enlarge the sample size but provide a novel insight into common neurobiological substrates as well. Abnormal activation pattern identified in the insula, IPL and precuneus implicated that dysfunction of goal-directed attention and action11,12, emotional processing and regulation13,14 in high-risk relatives. Functional decoding and decomposition consistently confirmed the dominating position of the emotion domain in high-risk relatives at the whole-brain level. Subgroup findings further suggested specific abnormal activation pattern associated with disorder, experiment and age. Longitudinal developmental studies are prioritized to further disclose the biomarker of genetic vulnerability in mood disorders.Conclusions
In conclusion, this voxel-wise meta-analysis implicates that relatives of patients with MDD and BD shared abnormal functional activation pattern in the precuneus, parietal and insular cortex. Among these regions, hyper-activated insular and surrounding limbic areas exhibited more specificity towards relatives of BD patients, while mild hypo-activated parietal, precuneus and surrounding default mode network appear to be MDD-specific. Although deficits of multiple behavioral domains in the high-risk relatives, functional decoding suggests that the genetic vulnerability of mood disorders might be primarily related to dysfunction in emotion domain. These neurofunctional correlates provide a novel insight into intermediate phenotype for mood disorders and have the potential to serve as preventative targets for protecting high-risk population from disease development.Acknowledgements
No acknowledgement found.References
1. Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137-158.
2. Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013;381:1654-1662.5.
3. Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484-503.
4. Cattarinussi G, Di Giorgio A, Wolf RC, et al. Neural signatures of the risk for bipolar disorder: A meta-analysis of structural and functional neuroimaging studies. Bipolar Disord. 2019;21:215-227.
5. Luking KR, Pagliaccio D, Luby JL, et al. Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry. 2016;55:328-337.
6. Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365-376.
7. Krueger RF, Eaton NR. Transdiagnostic factors of mental disorders. World Psychiatry. 2015;14:27-29.
8. Janiri D, Moser DA, Doucet GE, et al. Shared Neural Phenotypes for Mood and Anxiety Disorders: A Meta-analysis of 226 Task-Related Functional Imaging Studies. JAMA Psychiatry. 2020;77:172-179.
9. Albajes-Eizagirre A, Solanes A, Vieta E, et al. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage. 2019;186:174-184.
10. Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692-697.
11. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201-215.
12. Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9-16.
13. Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577-586.42. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242-249.
14. Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1-24.
Figures