1495
Functional and structural brain network features in Borderline Personality Disorder1Dipartimento di Fisica e Astronomia, Università di Bologna, Bologna, Italy, 2Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Bologna, Italy, 3IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
Synopsis
This study uses a graph-based approach to analyse the brain MRI data of early-stage Borderline Personality Disorder (BPD) patients. The topological properties of their Functional Connectivity and Structural Covariance weighted networks are explored. Compared to healthy controls, BPDs exhibit statistically significant alterations at global level, in terms of efficiency and modularity, and at local level, in centrality and efficiency, especially from the functional perspective. The nodal variations are mostly observed in the limbic system, in particular in those regions associated to emotion regulation. Such results may be anticipating structural alterations emerging in later stages of the disease.
Introduction
Borderline Personality Disorder (BPD) is a severe mental disorder, characterized by emotional and behavioural dysregulation (impulsivity, suicidality and self-harm), unstable sense of self, stress-related paranoid ideation or dissociative symptoms. Previous neuroimaging findings highlight the presence of brain alterations in this population; however, little is known about the topological organizations of brain networks. The present study aimed at describing functional and structural brain connectivity networks in a group of newly diagnosed BPD patients.Methods
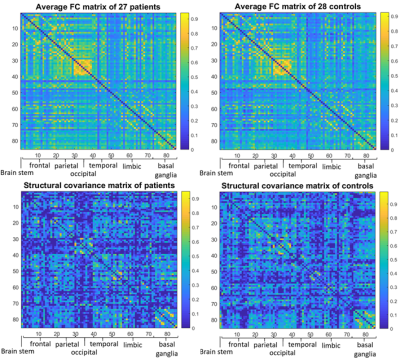
The brain MRI protocol (1.5T GE scanner) included resting-state functional (9 minutes, TR=3s) and T1-weighted structural images. Twenty-seven early-stage BPD patients recruited from community mental health centres (age: 24.1±3.5 years; females/males: 21/6) and twenty-eight healthy controls (HC, age: 24.9±2.8 years; females/males: 22/6) were enrolled. All participants also underwent a complete neuropsychiatric assessment.The pre-processed neuroimaging data were analysed with a graph-based approach. Brain images were parcellated into 85 cortical and subcortical Regions of Interest (ROIs) (Freesurfer, https://surfer.nmr.mgh.harvard.edu/) representing the nodes. A weighted Functional Connectivity Network (FCN) per subject was built by calculating the Pearson’s correlation between ROIs timeseries. The volume of each ROI was corrected for Total Intracranial Volume, sex and age, then a series was built with the volumes of all patients and controls separately and one weighted Structural Covariance Network (SCN) per group was constructed (Fig.1). Over the full range of graph densities (1-100%) a corresponding random network was generated, preserving the degree distribution, for comparison and normalization.
Additionally, two subnetworks were isolated for specific analysis: the limbic, being of particular interest for the disease, and the occipital, as a control. Moreover, a wavelet decomposition of the functional timeseries was performed to separately analyse the FCNs at four different ranges of frequencies (0.08-0.17, 0.04-0.08, 0.02-0.04, 0.01-0.02 Hz). Finally, structural networks retrieved using the data from female subjects only were also studied.
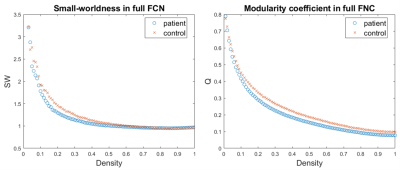
Networks topological properties were evaluated, using the Brain Connectivity Toolbox (https://www.nitrc.org/projects/bct/). At the global level, we evaluated the measures of efficiency of information transfer at long (global efficiency, characteristic path length) and short (average local efficiency, average clustering coefficient) range, the small-worldness, the largest connected component (LCC) size and the modularity coefficient. At the nodal level, centrality (degree, strength), information transfer efficiency (local efficiency, clustering coefficient), properties of integration (participation coefficient) and segregation (within-module strength) were examined. These measures were integrated over a range of densities specifically chosen for each type of network (basing on the small-worldness and LCC size) and the so obtained Areas Under Curve (AUCs) were compared between groups.
The statistical significance of the alterations was assessed using permutation tests (FDR-corrected p-Value<0.05, age and sex as nuisance variables). The significantly different properties were correlated (regressing-out sex, age and clinical condition) with the scores that participants obtained in the following neuropsychiatric scales: BIS-11 for impulsivity, DERS for difficulties in emotion regulation, ARS and RRS for rumination tendencies, SHI for self-harm tendencies, BDI for depression, WSAS for functional impairment in social context.
Results
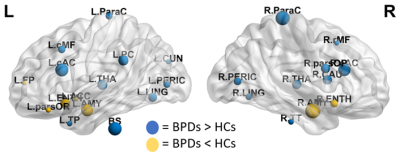
The FCNs showed differences between patients and controls both at global and local levels. Significant alterations emerged from the global measures of efficiency of information transfer at short range, LCC size and modularity, lower in patients (Fig.2). At nodal level, the variations mainly involved regions belonging to the fronto-limbic system, or in general having a role in emotion regulation. Among those, the amygdala and the entorhinal cortex (lower centrality and efficiency in BPDs), the caudal anterior cingulate cortex (higher centrality and efficiency in BPDs) and the left temporal pole (lower efficiency in BPDs) resulted to be particularly altered (Fig.3).The analysis of the wavelet-decomposed networks mainly showed altered properties at low frequencies (lower centrality of left temporal pole and right pars orbitalis in BPDs), and the comparison between the limbic and occipital subsystems confirmed considerable topological alterations only in the former.
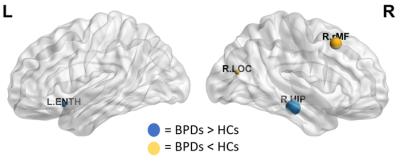
The results emerging from the SCNs were not as informative: at global level, no significant alterations were detected, while locally variations were mainly observed within the hippocampus (Fig.4) and the amygdala (females subjects only).
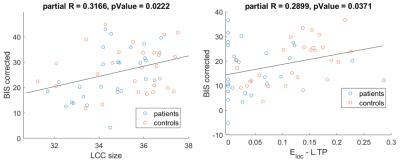
The exploratory analysis of the correlations revealed the existence of a relationship between specific neuropsychiatric symptoms (mostly impulsivity) with topological properties, both at global (LCC size) and nodal (centrality and efficiency of thalamus and temporal pole) (Fig.5).
Discussion
The results support the existence of a pathological involvement of the functional topological organization of the fronto-limbic system, and of regions typically involved in emotion regulation. These data are consistent with the literature investigating the brain activity in BPD patients1,2,3. Previous findings3 adopting a graph approach report opposite signs of the variations, however, since our patients were at an early stage of the disease, this might suggest that an evolution of the functional characteristics of the brain connectivity in BPDs may occur, as the illness progresses. The early stage of the disease might also explain why structural alterations did not emerge as clearly, as they might appear later during the course of the disease.Conclusions
The alterations in the brain connectivity of newly diagnosed BPD patients might anticipate structural modifications typically reported in literature. Therefore, it might be of interest to perform a longitudinal study of the topological properties of the brain at different stages of this disease.Acknowledgements
No acknowledgement found.References
1. Krause-Utz A. et al. Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol. Med. 2012; 42; 2181–2192.
2. Minzenberg M.J. et al. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007; 155; 241–243.
3. Xu T. et al. Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. NeuroImage: Clinical. 2016; 11; 302–315; doi:https://doi.org/10.1016/j.nicl.2016.02.006
Figures