1493
Investigation of Frontal Alpha Asymmetry EEG Neurofeedback in Major Depression Using Simultaneous fMRI1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Stephenson School of Biomedical Engineering, University of Oklahoma, Norman, OK, United States
Synopsis
We report a controlled study of emotion self-regulation training in patients with major depressive disorder (MDD) using frontal alpha asymmetry (FAA) EEG neurofeedback (EEG-nf) with simultaneous fMRI. MDD patients learned to significantly upregulate the FAA using EEG-nf while inducing happy emotion. Temporal correlations between the FAA and BOLD activity were significantly enhanced during the EEG-nf for many brain regions involved in emotion regulation, including the left DLPFC and the amygdala. Behavioral responses showed stronger approach bias after the training. Our study provides the first independent-modality evidence that the FAA-based EEG-nf can engage and influence the emotional brain circuitry.
Purpose
Modulation of frontal alpha EEG asymmetry (FAA) by means of EEG neurofeedback (EEG-nf) is a promising approach for emotion self-regulation training in major depressive disorder (MDD)1-4. By conducting EEG-nf training during fMRI, one can investigate BOLD fMRI correlates of the EEG-nf procedure and elucidate its mechanisms of action. Recent studies that employed FAA-based EEG-nf with simultaneous fMRI did not examine correlations between the FAA and BOLD activity and did not include a control group5,6. Here we report the first controlled study in which MDD patients used EEG-nf for upregulating the FAA during fMRI, and perform analyses of FAA-BOLD correlations. The results demonstrate that modulation of the FAA is accompanied by significant enhancements in temporal correlations between the FAA and BOLD activities of brain regions involved in emotion regulation.Methods
Fifteen right-handed unmedicated MDD patients completed the single-session EEG-nf study. The participants were randomly assigned to either an experimental group (EG, currently n=10) or a control group (CG, currently n=5). The EG participants were provided with FAA-based EEG-nf, while the CG participants were provided with sham feedback, unrelated to brain activity.GE MR750 3T MRI scanner with an 8-channel head coil was used for the experiments. A gradient-echo EPI sequence with FOV/slice=240/2.9 mm, TR/TE=2000/30 ms, SENSE R=2, image matrix 96×96, flip=90, 34 axial slices, was employed for fMRI. Simultaneous EEG recordings were performed using a 32-channel MR-compatible EEG system (Brain Products GmbH) with 0.1 µV resolution and 5 kS/s sampling, relative to FCz reference.
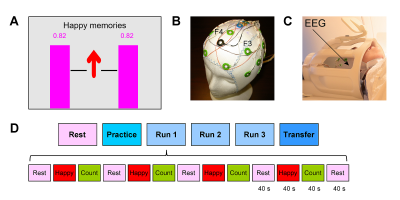
The EEG-nf (Fig. 1A) was implemented using a custom real-time multimodal data integration and control system7. The EEG-nf signal was displayed on the screen in the form of two identical variable-height magenta bars (Fig. 1A). The bar height was computed in real time as a change, with respect to a resting baseline, in relative alpha asymmetry A=(P(F4)−P(F3))/(P(F4)+P(F3)), where P is EEG power in the alpha band [7.5…12.5] Hz for channels F3 or F4 (Fig. 1B). The power was computed using FFT for a 4.096 s moving interval (4 ms sampling) with Hann window. The bar height was updated every 2 s. A custom procedure for improved real-time correction of EEG-fMRI artifacts was implemented in BrainVision RecView software as described previously8. It utilized a specially modified MR-compatible EEG cap equipped with four wire contours (Fig. 1B), providing reference artifact waveforms. Sham feedback signal was generated using the relative alpha asymmetry A computed for two reference artifact signals (from the larger contours on the left and on the right, after MR correction) instead of channels F3 and F4.
The experimental protocol (Fig. 1D) included six EEG-fMRI runs, and each run (except Rest) consisted of 40-s-long blocks of Rest, Happy Memories, and Count conditions8. For each Happy Memories with EEG-nf condition (Fig. 1A), a participant was asked to feel happy by evoking happy autobiographical memories, while trying to raise the level of the neurofeedback bars as high as possible. No bars were displayed during the Rest and Count conditions, and during the entire Transfer run. An Approach-Avoidance Task (AAT)9 with happy, neutral, and sad human face images was administered before and after the experiment.
Offline EEG data analyses were performed in BrainVision Analyzer 2, and fMRI analyses – in AFNI10. Normalized frontal alpha asymmetry was defined as FAA=ln(P(F4))−ln(P(F3)), and was computed for the individual upper alpha band8. The FAA time course was used to define EEG-based regressors for psychophysiological interaction (PPI) analyses of fMRI data as described previously4,8.
Results
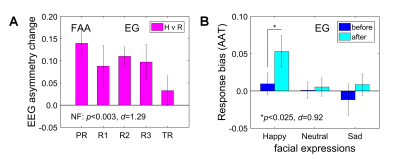
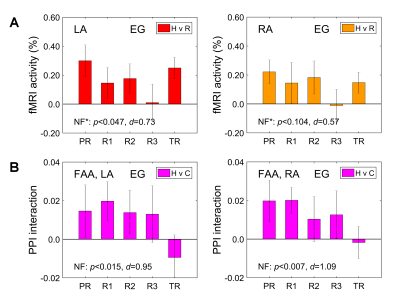
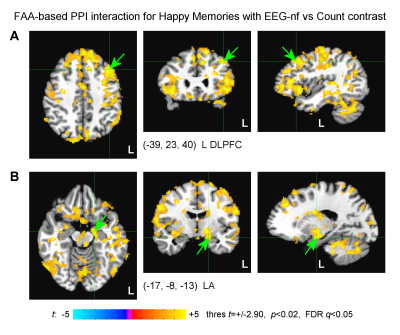
MDD patients in the EG were able to significantly upregulate the FAA using the EEG-nf (Fig. 2A). They also showed significant mood improvements and significant increase in approach-vs-avoidance response bias for happy faces after the experiment (Fig. 2B). The EEG-nf training was associated with significant fMRI activation of the left amygdala (LA) (Fig. 3A). The FAA-based PPI interaction effect for Happy Memories with EEG-nf vs Count condition contrast was positive and significant both for the LA and for the right amygdala (RA) ROIs (Fig. 3B). In the whole-brain analysis, the FAA-based PPI interaction effect was significant for regions of the emotion regulation network, notably the left dorsolateral prefrontal cortex (DLPFC, middle frontal gyrus, BA 8/9) (Fig. 4A). In the amygdala region, this effect was most pronounced for the superficial (SF) subdivision of the LA (Fig. 4B), consistent with previous studies4,8.Discussion
The EEG-informed fMRI analyses demonstrate that modulation of the FAA by means of the EEG-nf during happy emotion induction is associated with significant enhancements in temporal correlations between the FAA and BOLD activities of many brain regions involved in emotion regulation. These regions include, in particular, the left DLPFC, a major hub for cognitive control and approach motivation11, and the SF subdivision of the left amygdala, involved in reward processing and modulation of approach-avoidance behaviors12. Furthermore, MDD patients exhibit stronger approach bias in behavioral responses after the EEG-nf training, which suggests the possibility for correcting approach motivation deficits in depression13. These findings provide an independent evidence that the FAA-based EEG-nf can engage and influence the emotional brain circuitry.Acknowledgements
This work was supported by the Laureate Institute for Brain Research and the William K. Warren FoundationReferences
1. Baehr E, Rosenfeld JP, Baehr R. The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression: two case studies. J Neurotherapy 1997; 2:10-23.
2. Peeters F, Oehlen M, Ronner J, et al. Neurofeedback as a treatment for major depressive disorder – a pilot study. PLoS ONE 2014; 9:e91837.
3. Wang SY, Lin IM, Fan SY, et al. The effects of alpha asymmetry and high-beta down-training neurofeedback for patients with the major depressive disorder and anxiety symptoms. J Affect Disorders 2019; 257:287-296.
4. Zotev V, Yuan H, Misaki M, et al. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage Clin 2016; 11:224-238.
5. Cavazza M, Aranyi G, Charles F, et al. Towards empathic neurofeedback for interactive storytelling. OpenAccess Ser Informatics 2014; 41:42-60.
6. Dehghani A, Soltanian-Zadeh H, Hossein-Zadeh GA. Global data-driven analysis of brain connectivity during emotion regulation by electroencephalography neurofeedback. Brain Connect 2020; 10:302-315.
7. Zotev V, Phillips R, Yuan H, et al. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. NeuroImage 2014; 85:985-995.
8. Zotev V, Mayeli A, Misaki M, et al. Emotion self-regulation training in major depressive disorder using simultaneous real-time fMRI and EEG neurofeedback. NeuroImage Clin 2020; 27:102331.
9. Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: the Approach-Avoidance Task. Behav Res Therapy 2007; 45:2990-3001.
10. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162-173.
11. Spielberg JM, Miller GA, Engels AS, et al. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. NeuroImage 2011; 54:661-670.
12. Bzdok D, Laird AR, Zilles K, et al. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp 2013; 34:3247-3266.
13. Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emotion 2000; 14:711-724.
Figures