1492
Altered brain networks dynamics in first-episode drug-free schizophrenia1Huaxi MR Research Centre (HMRRC), Department of Radiology, West China Hospital of Sichuan University, chengdu, China
Synopsis
We investigated disturbances in dynamic functional connectivity (dFC) in whole-brain networks and altered dynamic functional topology of the brain regarding eigenvector centrality in first-episode drug-free patients with schizophrenia. By using resting-state functional magnetic resonance imaging (rf-MRI) data, schizophrenia was mainly manifested as prolonged dwell time in a state characterized by sparsely connected FCs and increased temporal variability of nodal centrality in the visual network, which may help us better interpret the mechanisms underlying visual and auditory hallucination in schizophrenia.
Introduction
Schizophrenia is a severe mental disease manifesting as delusion, hallucinations, apathy, and social withdrawal1. It is increasingly being conceptualized as a disorder that results from widespread dysconnectivity based on numbers of rf-MRI and graph-theory studies2,3. Recently, many studies have shown that the brain is not immutable but a dynamic complex system. Along with the development of dynamic methods, growing research interest on the brain’s temporal properties emerged and had demonstrated its effectiveness to reveal the neural mechanisms of schizophrenia. Therefore, the present study aims to investigate the dFC in whole-brain networks and dynamic functional topology of the brain in first-episode drug-free patients with schizophrenia.Methods
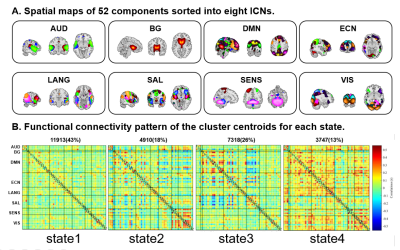
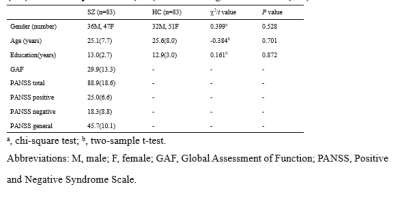
A total of 83 patients with schizophrenia and 83 matched healthy controls were enrolled in (Table 1). Rf-MRI data was obtained using a 3T GE MR scanner (TR/TE=2000ms/30ms, flip angle=90°, slice thickness=5mm, voxel size=3.75×3.75×5mm3). After data being preprocessed by DPARSF, group independent component analysis was used to obtain 52 meaningful independent components (ICs) which were identified as nodes and assigned to eight intrinsic connectivity networks (Figure 1A). Subsequently, sliding window approach was carried out to established dFC matrices with 20-TR window wide in steps of 1-TR. K-means clustering was performed to clustered dFC matrices into reoccurring states with optimal number according to a cluster number validity analysis. We quantified the group differences of FC strength in the same state and state alteration metrics (i.e. mean dwell time, fractional time) by two-sample t test and permutation test, respectively. To further explore the time-varying in topological centrality of each IC, eigenvector centrality (EC), a measure of centrality based on not only the strength of a region’s connections with other regions but also the importance of the connected brain regions themselves, was calculated for each component for each window. Alteration of EC dynamics was investigated with standard deviations (SD). A permutation test was applied in the comparison of SD of EC between groups, and significance was set at P<0.05 with false discovery rate (FDR) correction for all statistical analyses. Besides, verification inspection with 30-TR window was performed to check the result reliability.Results
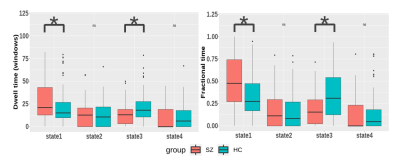
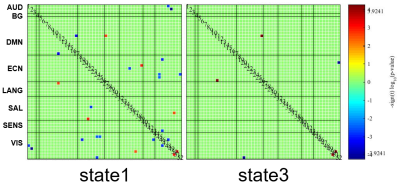
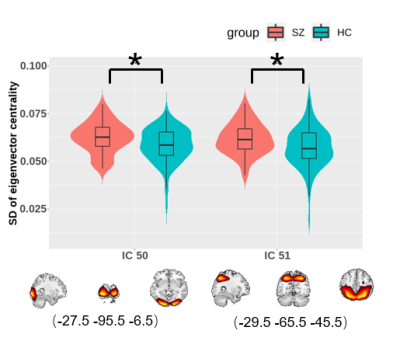
The clustering results determined four discrete states representing distinctive brain dynamic network configurations (Figure 1B). State1 occurred most frequently (43%) and the positive and negative FCs within and between networks were generally weak. State2 and state3 occurred with moderate frequency and both cluster centroids exhibited relatively strong positive intra-network FC and negative inter-network FC. State4 happened the least frequently (13%) while had extensive positive FCs within and between networks. We found that patients had more mean dwell times and fractional time than controls in state1 (P=0.0081 and P=0.0018) characterized by sparse FCs. Conversely, patients had fewer dwell times and fractional time in state3 (P=0.0018 and P=0.0009), and this state was characterized by moderate connection strength than controls (Figure 2). For the comparison of the FC strength in state1, we found decreased FC between the visual network (VN) and other networks and increased FC between language network (LN) and default-mode network (DMN) in patients than controls. In state3, patients showed decreased FC between VN and executive control network (ECN) (Figure 3). Further, in EC time-varying statistics, patients displayed increased temporal dynamics in two visual-related brain regions than controls (Figure 4). Those results can be repeated in the verification test.Discussion
Schizophrenia patients spent more time in a sparsely connected state (state1). This result suggests that a higher occurrence of a state with a sparse FC pattern in schizophrenia may explain previous findings of extensive dysconnectivity in static FC studies4. We also observed patients spent less time in state3, which might compensate for the more dwell time in state1 to balance the intrinsic dynamic function. In visual and auditory hallucination in schizophrenia, previous studies showed altered static FC in visual and language regions as well as abnormal interaction with DMN and ECN5-7. Therefore, the decreased FC strength between VN and ECN in state1 suggested that the visual hallucination might be presumably related to the dysregulation between executive control and visual perception networks. Moreover, the increased positive FC between LN and DMN in state1 in patients was opposite to the observation in healthy population, which had shown that the LN is functionally dissociated from the DMN8. In present study, the increased intrinsic FC between LN and DMN in state1 may help us better interpret the mechanisms underlying auditory hallucination in schizophrenia. In terms of EC dynamics, our findings of higher temporal variability of EC in two ICs in VN reflected decreased stability in the configuration of network communities during dynamic activity9 and might be relevant to the failure to provide stable coding of spatial location in schizophrenia10, which was consistent with the previously reported higher mean temporal variability in VN11.Conclusion
The present study found that patients with schizophrenia exhibited a higher occurrence of state 1 characterized by the sparse and weak FCs and increased dynamic variability (unstable dynamic activity) of regions in the visual network, which may play important roles in the visual perceptual impairments and auditory hallucination of schizophrenia. These findings could help unravel the mechanism in schizophrenia and probably provide new targets for treatment and intervention.Acknowledgements
Dr. Fei Li would like to acknowledge the support from the Sichuan Science and Technology Program (2019YJ0098).References
1.Yu Q, Erhardt E B, Sui J, et al. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: Application to healthy controls and patients with schizophrenia. Neuroimage, 2015; 107: 345-355.
2.Ray K L, Lesh T A, Howell A M, et al. Functional network changes and cognitive control in schizophrenia. Neuroimage Clin, 2017; 15: 161-170.
3.Sheffield J M, Barch D, Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev, 2016; 61: 108-20.
4.Dong D, Wang Y, Chang X, et al. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr Bull, 2018; 44(1): 168-181.
5.Alonso-Solís A, Vives-Gilabert Y, Grasa E, et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res, 2015; 161(2-3): 261-8.
6.Alderson-Day B, Diederen K, Fernyhough C, et al. Auditory Hallucinations and the Brain's Resting-State Networks: Findings and Methodological Observations. Schizophr Bull, 2016; 42(5): 1110-23.
7.Stephan-Otto C, Siddi S, Senior C, et al. Remembering verbally-presented items as pictures: Brain activity underlying visual mental images in schizophrenia patients with visual hallucinations. Cortex, 2017; 94: 113-122.
8.Mineroff Z, Blank I A, Mahowald K, et al. A robust dissociation among the language, multiple demand, and default mode networks: Evidence from inter-region correlations in effect size. Neuropsychologia, 2018; 119: 501-511.
9.Zhang J, Cheng W, Liu Z, et al. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain, 2016; 139(Pt 8): 2307-21.
10.Plomp G, Roinishvili M, Chkonia E, et al. Electrophysiological evidence for ventral stream deficits in schizophrenia patients. Schizophr Bull, 2013; 39(3): 547-54.
11.Deng Y, Liu K, Cheng D, et al. Ventral and dorsal visual pathways exhibit abnormalities of static and dynamic connectivities, respectively, in patients with schizophrenia. Schizophr Res, 2019; 206: 103-110.
Figures