1490
Sensitivity-enhanced and Shading-reduced Chemical Exchange Saturation Transfer Imaging of the Abdomen using Parallel Transmission1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Department of Radiology, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China, 3Techna Institute, University Health Network, Toronto, ON, Canada, 4MR Collaboration, Siemens Healthcare Ltd., Shanghai, China, 5MR Application Development, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China
Synopsis
CEST imaging benefits from longer saturation duration and a higher saturation duty cycle. Dielectric shading effects occur when the RF wavelength approaches the object size. Here, we proposed a parallel-transmission-based CEST (pTx-CEST) sequence to extend the maximum saturation duration at 100% duty-cycle and mitigate shading effects. The maximum saturation duration in pTx-CEST was lengthened to 2170, 3150, and 4130ms compared to 1050ms in non-pTx-CEST at TR of 3s, 4s, and 5s, respectively, leading to a significant sensitivity enhancement. Besides, the optimal amplitude ratio and phase difference between RF channels, manifesting circular or elliptical polarization, help reduce the dielectric shading effects.
Introduction
Chemical Exchange Saturation Transfer (CEST) imaging is capable of detecting endogenous low-concentration metabolites containing water-exchangeable protons 1-3. The CEST effects measured are partly determined by the factor of (1-exp(-tsaturaion/T1water)) 3, indicating that the longer the saturation duration, the more sensitive CEST imaging will be. However, clinical applications of CEST imaging are typically limited by either inadequate saturation duration 4,5 or low saturation duty cycles 6,7. In addition, dielectric shading artifacts occur when the RF wavelength approaches the size of the object 8, making it an obstacle for CEST imaging in the human abdomen. In this work, a parallel-transmission-based chemical exchange saturation transfer (pTx-CEST) imaging sequence is proposed to achieve arbitrarily long saturation duration and a 100% duty cycle simultaneously for enhanced CEST sensitivity, as well as to mitigate dielectric shading artifacts in CEST imaging.Methods
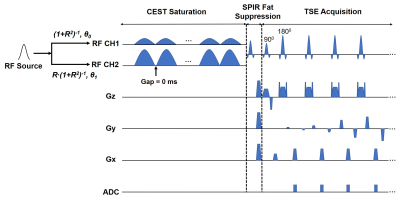
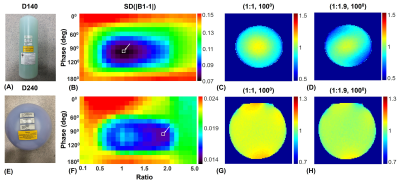
The study was performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 2-channel body coil for RF transmission. The two transmit channels in the CEST saturation module can be driven with arbitrary amplitude ratios R and phase differences (θ1 - θ0), independently, using freely-defined external RF shapes through the vendor-provided pTx functionality (Fig. 1). Comparison between the proposed pTx-CEST and the conventional non-pTx-CEST sequences was performed in a bovine serum albumin (BSA) phantom consisted of a flask with 2% agarose gel and two test tubes filled with 10% and 5% BSA dissolved in phosphate-buffered saline (PBS). The RF pulse shape and peak transmit voltage in the pTx-CEST sequence were the same as those in the non-pTx-CEST sequence. Optimizations of amplitude ratio and phase difference settings for best B1 homogeneity measured with a pre-conditioning RF method 9 were performed on a 140-mm-diameter (D140) cylindrical phantom filled with NiSO4∙6H2O and NaCl solution, and a 240-mm-diameter (D240) spherical phantom filled with Marcol oil. The two vendor-provided phantoms were chosen to mimic the size of the human brain and abdomen. Scans on the D140 and D240 phantoms were performed using a 20-channel head coil and an 18-channel flexible coil for signal reception, respectively. The uniformity of the B1 field map was calculated with the metric: SD(|B1-1|), where SD referred to standard deviation. The optimal amplitude and phase setting yielding the best homogeneity in the phantoms was used in the human study of pTx-CEST scans. The in vivo study was approved by the local Institutional Review Board, and written consent forms were obtained from the volunteers recruited.Results
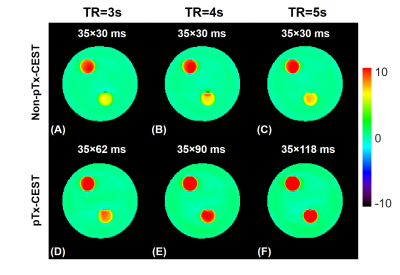
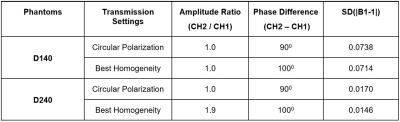
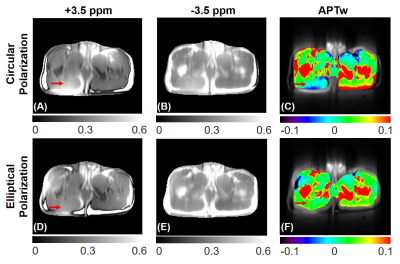
Fig. 2 demonstrates the maximum saturation duration with 100% duty cycle was significantly lengthened to 2170, 3150, and 4130 ms using the pTx-CEST sequence compared to 1050 ms in the non-pTx-CEST sequence at the TR of 3s, 4s, and 5s, respectively. The amide-proton-transfer-weighted (APTw) signals in the BSA tubes obtained from pTx-CEST were substantially higher than those derived from non-pTx-CEST, indicating a remarkable sensitivity enhancement by elongating the saturation duration. For the metric concerning B1 homogeneity, the optimal settings revealed essential circular polarization (CP) with an amplitude ratio of 1 and a phase difference of 1000 between channels in the D140 phantom (Fig. 3A-D), and elliptical polarization (EP) with an amplitude ratio of 1.9 and phase difference of 1000 between channels in the D240 phantom (Fig. 3E-H). Table 1 shows the comparison of homogeneity metrics from circular and elliptical polarization, which was in line with the previous studies 10,11. Consequently, the EP mode was used in CEST imaging of the human abdomen because of its similar size to the D240 phantom. The regions with abnormally high intensity in muscle tissues of the image acquired at +3.5 ppm with non-pTx-CEST (Fig. 4A, red arrow), possibly due to the dielectric shading effect, disappeared in pTx-CEST (Fig. 4D, red arrow). Accordingly, the extremely low in the APTw images of the non-pTx-CEST sequence vanished compared to that in the pTx-CEST sequence (Fig. 4C, F, red arrows).Discussion & Conclusion
We proposed a pTx-CEST sequence to extend the maximum saturation duration with a 100% duty cycle, which significantly enhanced the CEST imaging sensitivity compared to the conventional non-pTx-CEST sequence. The optimal setting for best B1 homogeneity of the two transmit channels exhibited elliptical polarization when the object's size approached the RF wavelength. Experiments regarding optimizations for B1 homogeneity were performed in phantoms in place of human brains and abdomens because of the excessively long scan duration and subject-level variations. It is worthwhile noting that we only switched the CEST saturation module from the non-pTx mode to the pTx-mode without changing the RF pulses in the readout sequence. Importantly, the maximum saturation duration in the non-PTx mode was not able to be increased when the TR changed from 3s to 5s. Thus, the limited maximum saturation duration in the non-pTx mode was probably due to certain software restriction, which was bypassed in pTx functionality. Nevertheless, running the pTx-CEST sequence was still found to be monitored by the SAR safety watchdog in our practical scans. In conclusion, this study demonstrated the feasibility of arbitrarily long CEST saturation duration with 100% duty cycle using the pTx functionality on the Siemens platform, and the RF settings for optimal B1 homogeneity varied depending on the target size.Acknowledgements
NSFC grant number: 81971605, 61801421.References
1. Ward K, Aletras A, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143(1):79-87.
2. Zhou J, van Zijl PC. Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc 2006;48(2-3):109-136.
3. Van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011;65(4):927-948.
4. Zhou J, Blakeley JO, Hua J, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 2008;60(4):842-849.
5. Zhu H, Jones CK, van Zijl PC, et al. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn Reson Med 2010;64(3):638-644.
6. Schmitt B, Zaiß M, Zhou J, et al. Optimization of pulse train presaturation for CEST imaging in clinical scanners. Magn Reson Med 2011;65(6):1620-1629.
7. Sun PZ, Lu J, Wu Y, et al. Evaluation of the dependence of CEST-EPI measurement on repetition time, RF irradiation duty cycle and imaging flip angle for enhanced pH sensitivity. Phys Med Biol 2013;58(17):N229.
8. Hoult DI. Sensitivity and power deposition in a high‐field imaging experiment. J Magn Reson Imaging 2000;12(1):46-67.
9. Chung S, Kim D, Breton E, et al. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64(2):439-446.
10. Nistler J, Diehl D, Renz W, et al. Homogeneity improvement using a 2 port birdcage coil. ISMRM 2007.
11. Murbach M, Neufeld E, Cabot E, et al. Virtual population‐based assessment of the impact of 3 Tesla radiofrequency shimming and thermoregulation on safety and B1+ uniformity. Magn Reson Med 2016;76(3):986-997.
Figures