1486
Golden-Angle Radial CEST MR Fingerprinting with Temporal Compressed Sensing Reconstruction1Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Chemical Exchange Saturation Transfer (CEST) is a novel imaging technique that is sensitive to metabolite concentrations at imaging resolutions in clinically relevant scan times. These features have generated much interest and applications of CEST in assessing disease pathologies, progression and therapeutic response are currently being explored. A quantitative framework for CEST based on MR Fingerprinting (MRF) was recently proposed using an EPI readout. Here we describe preliminary work to leverage the inherent B0 and motion robustness of radial imaging to develop a clinical golden-angle radial CEST-MRF that doesn't suffer from the classic EPI drawbacks.
Introduction
Chemical Exchange Saturation Transfer (CEST) is a novel imaging technique that is sensitive to metabolite concentrations at imaging resolutions in clinically relevant scan times [1]. These features have generated much interest and applications of CEST in assessing disease pathologies, progression and therapeutic response are being explored in various pathologies [2,3]. Nevertheless, conventional CEST is a non-quantitative technique and also suffer from long scan times. Recently, a CEST pulse sequence based on the MR Fingerprinting (MRF) framework was described and demonstrated in the mouse brain [4,5] using an EPI readout. EPI suffers from well-known shortcomings like long echo times and susceptibility induced geometric distortions and is not suited for body imaging. In this work we describe preliminary work to leverage the inherent B0 and motion robustness of radial imaging to develop a clinical golden-angle radial CEST-MRF sequence and a temporal compressed sensing reconstruction for multiparametric quantitative body MRI. We demonstrate the initial proof-of-concept with phantom experiments.Methods
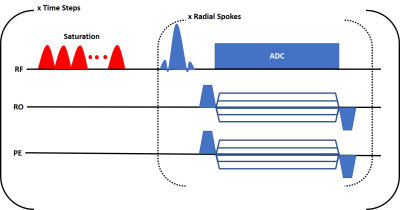
Pulse SequenceAll experiments were conducted on a GE Signa Premier (GE Medical Systems, Milwaukee, WI, USA) 3T scanner with a 48-channel head receiver coil. A fast gradient echo pulse sequence was modified (Figure 1) to include a saturation pulse train before k-space sampling. The pulse train consisted of 160 gaussian shaped pulses of 16 ms duration with a variable, user-defined frequency. k-space was sampled with golden-angle radial spokes to maximize incoherence between time step frames [6]. 16 spokes were acquired for each frame and each spoke contained 256 readout points. The acquisition parameters were as follows: repetition time (TR)=3500 ms, flip angle (FA)=20°, saturation time (Tsat)=2560 ms, saturation power (B1)=4 uT, echo time (TE)=5 ms. The field of view was 240 mm2 with a slice thickness of 5 mm.
Phantom
The sequence was tested in the GE “Braino” phantom (GE Medical Systems, Milwaukee, WI, USA) which includes 10 mM of creatine [7 ]whose exchangeable protons resonate at ~1.8ppm.
Z spectrum
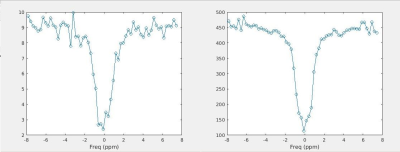
To demonstrate the utility of our sequence, a Z spectrum was acquired by varying the saturation pulse frequency from time step to time step. The saturation frequency was varied from -8 to 8ppm in increments of 0.27 ppm for a total of 60 resonance offsets. The scan length for this acquisition was 3.5 minutes.
CEST-MRF
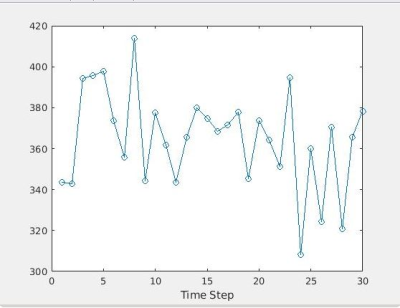
To demonstrate the potential of the sequence for CEST-MRF acquisitions the saturation pulse power was varied for each time step as shown in Figure 1 while the saturation frequency was set to a constant 1.8ppm. All other parameters were kept constant. The acquisition of the 30 time steps required 105 seconds.
Image Reconstruction
The time-series of undersampled golden-angle k-space data was reconstructed using the GRASP algorithm [6] to remove aliasing artifacts before quantification. GRASP jointly exploits sparsity in the difference between temporal frames and parallel imaging.
Results
Figure 3 shows the Z spectrum obtained using the proposed pulse sequence and reconstruction pipeline in comparison to simple NUFFT reconstruction. The GRASP reconstruction significantly reduced the noise in the Z spectrum and renders visible the slight flattening of the curve due to the creatine resonance at 1.8 ppm. The CEST-MRF plots are shown in Figure 4 and manifest the expected signal intensity variations.Discussion/Conclusion
A flexible CEST-MRF pulse sequence based on golden-angle radial sampling was developed. Radial sampling is attractive because it allows for substantially shorter repetition times compared with EPI sampling commonly used in CEST sequences which reduces its sensitivity to B0 inhomogeneities. In addition, the inherent motion robustness of radial sampling makes it more suitable for body imaging where cardiac and respiratory motion are a challenge. Iterative reconstruction methods like the GRASP technique used in this work enable high acceleration factors while still providing good image quality. For quantitative applications such as MRF, care must be taken to ensure that the regularization in the reconstruction doesn’t affect the signal intensity and hinder accurate quantification. This will be explored in future work. Also left for future work is demonstration of the sequence in in vivo human applications.Acknowledgements
No acknowledgement found.References
1. Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, Mu L, Nanz D, Tuura R, Delso G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. Springer; 2016;3(1):19.
2. Ma B, Blakeley JO, Hong X, Zhang H, Jiang S, Blair L, Zhang Y, Heo H-Y, Zhang M, van Zijl PC, others. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J Magn Reson Imaging. 2016;44(2):456–462.
3. Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. AACR; 2017;23(14):3667–3675.
4. Cohen O, Huang S, McMahon MT, Rosen MS, Farrar CT. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn Reson Med. Wiley Online Library; 2018;80(6):2449–2463.
5. Perlman O, Ito H, Herz K, Shono N, Nakashima H, Zaiss M, Chiocca EA, Cohen O, Rosen MS, Farrar CT. AI boosted molecular MRI for apoptosis detection in oncolytic virotherapy. bioRxiv. Cold Spring Harbor Laboratory; 2020;
6. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. Wiley Online Library; 2014;72(3):707–717.
7. Lattanzi R, Grant AK, Polimeni JR, Ohliger MA, Wiggins GC, Wald LL, Sodickson DK. Performance evaluation of a 32-element head array with respect to the ultimate intrinsic SNR. NMR Biomed Int J Devoted Dev Appl Magn Reson Vivo. Wiley Online Library; 2010;23(2):142–151.
Figures