1485
B0 and B1 correction anti-respectively for chemical exchange saturation transfer imaging1MR Collaboration, Siemens Healthcare Ltd., Shanghai, China, 2Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
We propose a new simultaneous B0 and B1 correction for Chemical Exchange Saturation Transfer imaging. This method undersamples the number of offsets on the B1 and B0 plane. The CEST map's mean error almost halved at the upper brain region compared with the conventional method and the minimal sampled B0/B1 correction method. The proposed method provides an efficient B0 and B1 correction and may improve the diagnostic accuracy using CEST imaging at 3T.
Introduction
Chemical Exchange Saturation Transfer(CEST) detects dilute solute in-vivo via water signal through the exchange of saturated proton from the solute. CEST has been used to grade glioma1, lateralize epileptic foci2 and predict proteoglycan level3. However, the CEST values itself depend on the B1 strength. The influence of B1 has been corrected mostly at 7T but not at 3T. The uncorrected CEST map may jeopardize the accuracy of diagnosis. Traditionally B0/B1 is corrected by performing first the B0 correction followed by B1 correction4. Thereby the exact same offsets have to be acquired per B1 value. Here, we proposed a novel method that implements B0 and B1 correction anti-respectively (B0B1CAR) and apply this method for amide proton transfer weighted (APTw) imaging at 3T. The proposed method has higher flexibility in data sampling at different B1 and RF offsets. Our results demonstrate that compared with the conventional method (B0 only correction) and the B0/B1 correction with minimal sampled data, the proposed method halved the upper brain region's error with the cost of little increase of scan time.Methods
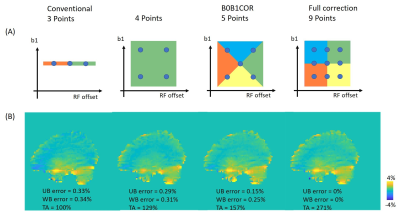
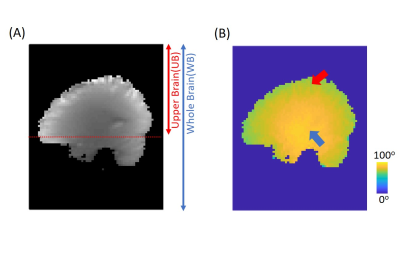
Figure 1A illustrates the conventional B0/B1 correction method. Step 1, the data at the target frequency offset (red dots) were interpolated for each sampled B1 value separately. Step 2, the data at the target frequency offset and target B1 (green dot) was interpolated by the B0 corrected data from step 1. Figure 1B illustrates the B0B1CAR method for simultaneous B0 and B1 correction. The target frequency offset and B1 data were interpolated directly by sampled data at different frequency offsets and B1. This method has no restriction on data sampling patterns at RF offsets and B1 values. We used SPACE to collect CEST data5 (TR = 3000ms, TE = 3.6ms, ETL = 165, Averages = 1.4, GRAPPA = 2 X 2, resolution = 2.75 mm3, 10 X 90ms Gaussian pulses, 10ms gap). B1 map was collected by a pre-saturated tfl method. Multi-echo GRE with the same FOV and resolution was used to generate a field map. After correction, the data at frequency offset = ±3.5ppm, and B1 = 2μT were used to calculate APTw images by MTR asymmetry6. A volunteer was scanned on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Figure 2A illustrates the data sampling and correction method in this study. Blue dots represent the sampled data at different RF offsets and B1, and regions with different colors were interpolated from different sets of points. The conventional method is a B0 only corrected method that is widely used6. For correcting B1, a 9 points method was used with RF offsets = -300, ±3, ±3.5, ±4 ppm and a nominal B1 of 1.6, 2, 2.4 μT. The acquired data for the other methods can be derived from Figure 2A. Mean errors were calculated for the upper brain region (UB, Figure 3A) and the whole brain region (WB) as the mean absolute difference between each method and the full correction method. The acquisition time (TA) was compared with the conventional method, which has a 100% acquisition time.Results
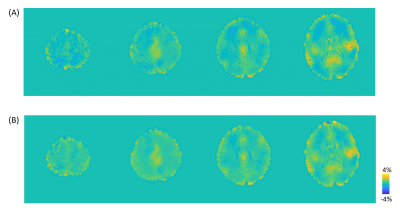
Figure 3B shows the measured flip angle map for nominal 90° flip angle. The peripheral brain region is about 70°(red arrow), and the central region is close to 90°(blue arrow). Figure 2B shows the APTw images with B0/B1 correction by methods illustrated in Figure 2A. The conventional 3 points method has the largest mean errors. The 4 points method, which used the minimum points required for B0/B1 correction, has smaller errors at the cost of 129% scan time. The B0B1CAR method has less than 50% error (0.33% -> 0.15%) in the upper brain region compared with the conventional method but less improvement for the whole brain region. The full correction method was used as a reference to quantify the goodness of correction. It used a 271% acquisition time compared with the conventional method. Figure 4 shows the sagittal view of APTw images corrected by the conventional method and B0B1CAR. The peripheral brain region of conventional APTw images shows a lower value due to a lower B1 value.Discussion
The conventional 3 points method was not correct for the B1 inhomogeneity and the low B1 value caused a higher error in APTw images near the peripheral brain region. The 4 points method used the minimal sampled data for both B0 and B1 correction, but the error only reduced marginally compared with the conventional method. Compared with the 4 points method, B0B1CAR acquired one more data point for B0/B1 correction, and the upper brain's error almost halved. However, the error in the whole brain region was only reduced by 0.06%. We hypothesize that the B0B1CAR with only 5 points was not enough for the region with high off-resonance.Conclusion
The proposed B0B1CAR method provides a more flexible data sampling method for simultaneous B0 and B1 correction in CEST imaging. We demonstrate that B0B1CAR with 5 points can correct for B0 and B1 artifact efficiently in the upper brain region with almost halved error, and only one more data is required on each side of the z spectrum than the 4 points method.Acknowledgements
No acknowledgement found.References
1. Togao, O. et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-oncology 16, 441–448 (2014).
2. Davis, K. A. et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Science translational medicine 7, 309ra161–309ra161 (2015).
3. Schmitt, B. et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and 23Na MR imaging at 7 T. Radiology 260, 257–264 (2011).
4. Singh, A., Cai, K., Haris, M., Hariharan, H. & Reddy, R. On B1 inhomogeneity correction of in vivo human brain glutamate chemical exchange saturation transfer contrast at 7T. Magnetic resonance in medicine 69, 818–824 (2013).
5. Zhang, Y. et al. Whole-brain chemical exchange saturation transfer imaging with optimized turbo spin echo readout. Magnetic Resonance in Medicine (2020).
6. Zhou, J. et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 60, 842–849 (2008).
Figures