1477
High Resolution Adiabatic T1ρ Mapping Using 3D MAPSS Sequence at 3T1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Philips Healthcare, Andover, MA, United States, 3Department of Radiology, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, United States
Synopsis
The 3D MAPSS T1ρ sequence provides fast and accurate T1ρ quantification with high spatial fidelity using RF phase cycling and variable flip angle in a MP-GRE sequence. However, the conventional 3D MAPSS sequence uses continuous-wave RF pulses for spin locking and is thus sensitive to B0 and B1 field inhomogeneities. In this study, a 3D MAPSS T1ρ sequence with adiabatic RF pulses was implemented and was shown to be less sensitive to B0 frequency offset on phantom compared to conventional 3D MAPSS T1ρ mapping. It was also successfully applied to high-resolution 3D T1ρ mapping of knee cartilage and brain tissue.
INTRODUCTION
Quantitative T1ρ mapping has gained increasing attention due to its high sensitivity to low-frequency molecular motion process that allows characterization of macromolecular composition and proton exchange in tissue.1 Standard T1ρ preparation uses a composite continuous-wave (CW) RF pulse for spin locking, but it is relatively sensitive to B0 and B1 imperfections. Self-compensating spin lock pulses and 180° refocusing pulses can be used in the preparation to partially compensate for B0 and B1 inhomogeneities to reduce banding artifacts, but the off-center slices are still subject to quantification inaccuracy. 3D Magnetization-Prepared Angle-Modulated Partitioned k-Space Spoiled Gradient Echo Snapshots (3D MAPSS) is a fast MP-GRE technique using RF phase cycling and a variable flip angle train to achieve fast T1ρ mapping with high accuracy and spatial fidelity.2 Its quantification accuracy however is compromised by the CW-T1ρ preparation. Instead, adiabatic RF pulses with both amplitude and frequency modulations, such as the hyperbolic secant pulses (HSn, n = 1, 2, 4, 8), have a wider bandwidth and are much less sensitive to B0 and B1 inhomogeneities, as shown in some previous studies.3,4 The purpose of this study was to implement a 3D MAPSS sequence with adiabatic T1ρ preparation to achieve fast, accurate quantification with high spatial resolution. The sequence was tested on phantom, human knee and brain at 3T.METHODS
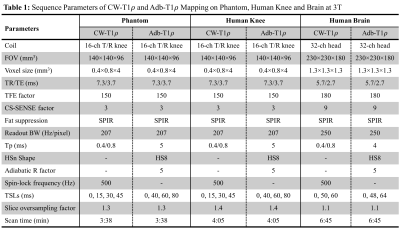
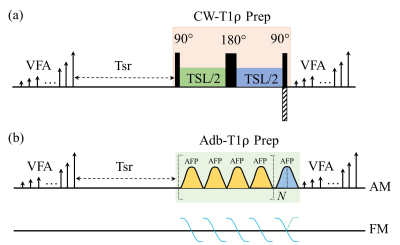
Figure 1 illustrates the 3D MAPSS T1ρ sequences with continuous-wave (Figure 1a: CW-T1ρ) and adiabatic (Figure 1b: Adb-T1ρ) RF pulses used for spin locking. CW-T1ρ preparation was implemented as a composite RF pulse consisting of 90°-TSL/2-180°-TSL/2-90°, where TSL is the time of spin locking. Adiabatic full passage (AHP) RF pulses with HS8 amplitude and frequency modulations were used for Adb-T1ρ preparation. A group of four AHP pulses following MLEV phase cycling were used for spin locking. For CW-T1ρ, the last 90° hard pulse was used for phase cycling by reversing its phase for two paired scans. For Adb-T1ρ, phase cycling was achieved by reversing the frequency modulation for the second half of the last AFP pulse. Both cases lead to a pair of scans with same TSL but opposite longitudinal magnetization (Mz+ or Mz-) immediately before data acquisition. A complex subtraction of data from the paired scans can eliminate the contaminating signal from T1 relaxation.2 CW-T1ρ and Adb-T1ρ experiments were performed on phantom (three pairs of tubes with 2%, 3% and 4% homogeneous agarose concentrations), human knee and brain (male healthy volunteer, 45 years old) on a 3T clinical scanner (Ingenia Elition, Philips Healthcare, The Netherlands). Table 1 summarizes the sequence parameters for each experiment. T1ρ maps were calculated using a mono-exponential two-parameter fitting algorithm. The center slice (slice 24) and two off-center slices (slices 8 and 41) of the phantom were selected for quantification using a custom analysis tool developed in IDL (Exelis VIS, Boulder, CO). The study was approved by local institutional review board and consent was received from the participating subject.RESULTS
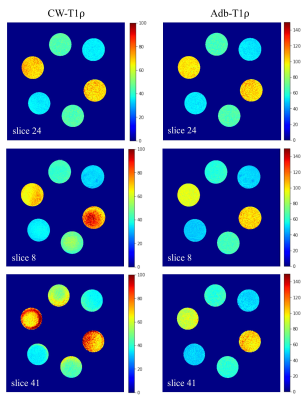
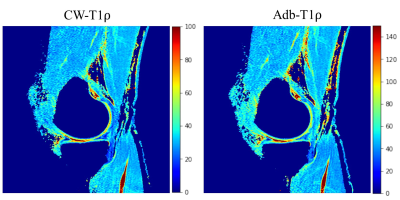
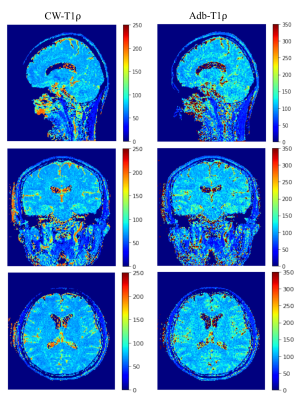
Figure 2 shows T1ρ maps of the phantom at the center slice (slice 24) and two off-center slices (slices 8 and 41) where frequency offset is present. The T1ρ maps of center slice look similar and are free of artifacts (Figure 2, top row) for both CW-T1ρ and Adb-T1ρ. Severe artifacts can be observed in the two off-center slices for CW-T1ρ (middle and bottom left), indicating that Adb-T1ρ is less sensitive to B0 field inhomogeneities. Quantitative results further confirmed that Adb-T1ρ provides more consistent T1ρ measurements in the off-center slices compared to CW-T1ρ. For the center slice (slice 24), both CW-T1ρ and Adb-T1ρ provided very consistent T1ρ values (largest difference < 2%) for tubes with the same agarose concentration. However, for slice 8, CW-T1ρ values were (38.21±1.28 ms, 34.33±1.19 ms), (53.23±2.28 ms, 43.59±1.25 ms), (83.41±7.85 ms, 65.62±3.17 ms) for the three pairs of tubes, indicating significant variation of the T1ρ values (largest difference = 27%) for tubes with same agarose concentration. In contrast, the T1ρ values from Adb-T1ρ were more consistent as follows, (49.00±1.72 ms, 51.45±1.96 ms), (67.55±2.45 ms, 63.16±2.09 ms), (100.32±5.22 ms, 95.89±2.95 ms) for three pair of tubes (largest difference = 7%). Similar observations were found for slice 41. Figure 3 shows good delineation of the knee cartilage for both CW-T1ρ and Adb-T1ρ maps without apparent artifacts or boundary blurring. Figure 4 illustrates example CW-T1ρ and Adb-T1ρ maps with isotropic resolution of the brain in sagittal (top row), coronal (middle row), and axial (bottom row) views.DISCUSSION
3D MAPSS T1ρ mapping with adiabatic RF pulses was successfully implemented and the phantom experiment demonstrated that Adb-T1ρ is less sensitive to frequency offset compared to CW-T1ρ, suggesting Adb-T1ρ may provide more accurate T1ρ measurement in conditions where magnetic field is inhomogeneous. The Adb-T1ρ sequence also provided good image quality for human knee and brain T1ρ mapping. Future study is warranted to quantitatively compare the performance of Adb-T1ρ to conventional CW-T1ρ in the presence of B0 and/or B1 inhomogeneities in human knee and brain applications.CONCLUSION
3D MAPSS Adb-T1ρ offers fast and high resolution T1ρ mapping as 3D MAPSS CW-T1ρ and is more robust to B0 inhomogeneities to potentially obtain more accurate T1ρ quantification.Acknowledgements
None.References
1. Wang Y, Zhang Q, Li X, Chen W, Ahuja A, Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg 2015; 5(6): 858-885.
2. Li X, Han ET, Busse RF, Majumdar S. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med. 2008; 59(2):298-307.
3. Schuenke P, Koehler C, Korzowski A, Windschuh J, Bachert P, Ladd ME, Mundiyanapurath S, Paech D, Bickelhaupt S, Bonekamp D, Schlemmer H, Radbruch A, Zaiss M. Adiabatically prepared spin-lock approach for T1ρ-based dynamic glucose enhanced MRI at aultrahigh fields. Magn Reson Med 2017; 78(1):215-225.
4. Okuaki T, Takayama Y, Nishie A, Ogino T, Obara M, Honda H, Miyati T, Van Cauteren M. T1ρ mapping improvement using stretched-type adiabatic locking pulses for assessment of human liver function at 3T. Magn Reson Imaging 2017; 40:17-23.
Figures