1462
Highly Accelerated 1mm3-Isotropic 3D CEST MRI with Spectral Random Walk CAIPIRINHA Sampling at 7T
Sugil Kim1, Seong-Gi Kim2,3, and Suhyung Park4,5
1Siemens Healthineers, Seoul, Korea, Republic of, 2Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of
1Siemens Healthineers, Seoul, Korea, Republic of, 2Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 5Department of ICT Convergence System Engineering, Chonnam National University, Gwangju, Korea, Republic of
Synopsis
We propose highly accelerated 3D CEST MRI using CAIPI sampling based 3D segmented EPI with spectral random walk, potentially enabling 1mm-isotropic whole-brain CEST imaging within 5min at 7T.
Introduction
Chemical Exchange Saturation Transfer (CEST) Magnetic Resonance Imaging (MRI) has been playing an important role in molecular MRI providing distinct contrast based on proton exchange mechanism in metabolic, molecular, and cellular information in tissue [1-2]. At ultra-high fields, CEST MRI holds great potentials by improving the sensitivity to CEST contrast between the ischemic and normal areas. However, the direct saturation effect makes CEST MRI very sensitive to B0 inhomogeneity, thus requiring multiple acquisitions called z-spectrum for B0 correction. The acquisition time becomes prohibitively prolonged particularly in case of volumetric imaging. In this work, we propose highly accelerated 3D CEST MRI using CAIPI sampling based 3D segmented EPI with spectral random walk, potentially enabling 1mm-isotropic whole-brain CEST imaging within 5min at 7T.Method
3D Segmented Pulse Sequence with Spectral Random Walk for 3D CEST MRIA schematic of 3D CEST MRI pulse sequence is shown in Fig. 1a. Gaussian-shaped RF pulses are applied with multiple frequency offsets to generate CEST contrast. Segmented 3D EPI are then successively applied with CAIPI sampling to reduce g-factor noise penalty. To further improve the encoding power across z-spectra, the CAIPI sampling is combined with spectral random walk by randomly shifting the spatial CAIPI sampling within a ky-kz block (the block size is under-sampling factor for each dimension) along the spectral dimension, leading to spectrally random encoding while keeping overall spatial CAIPI pattern unchanged (Fig. 1b). Additionally, we employ a liner ordering along the kz direction to minimize CEST contrast bias that arises from signal fluctuation before reaching to the steady-state.
With a new sampling scheme, we exploit the spectral redundancy by incorporating all available information into constrained reconstruction framework unlike the conventional segmented 3D EPI that operates in a time-independent manner, reconstructing images individually. To this end, All image are reconstructed using: argminx||FEx-y||+λ1||Wx||1 +λ2||TV(x)||1 where F is the Fourier transform, E is encoding matrix with coil sensitivities, y is the measured k-space data, W is the sparsifying transform, TV is the total variation, λ1 and λ2 are regularization parameters , x is the under-sampled data to be reconstruction [5].
Experimental Study
The imaging parameters in the data were as follows: Matrix size=192x144x192(ky, kz, kx), FOV=192x158x192, 1x1x1.1mm, flip angle=10 degrees, TR=65ms, TE=23ms, EPI factor=29, partial Fourier ky direction 7/8. For all experiments are acquired with following CEST parameters: CEST RF amplitude 0.7uT, duration 30ms, and 51 saturation frequencies (±300, ±10, ±7, ±6, ±5.5, ±5, ±4.75, ±4.5, ±4.25, ±4, ±3.75, ±3.5, ±3.25, ±3, ±2.75, ±2.5, ±2.25, ±2, ±1.75, ±1.5, ±1.25, ±1, ±0.75, ±0.5, ±0.25, 0), total imaging time=5min 18sec. We used a 7T scanner (Magnetom Terra, Siemens Healthineers, Erlangen, Germany) with a 32-channel heal coil. B0 correction was applied WASSR [6] technique. To efficiently suppress fat signal, we utilized a non-selective rectangular RF pulse by selectively exciting water-only signal [4].
Results and Discussion
Fig. 2(a) shows reconstructed (unsaturated) images acquired from the proposed acquisition strategy. Noted that the reconstructed imaging volume robustly remove noises and aliasing artifacts from highly undersampled data by exploiting spectral redundancy. Fig. 2(b) shows the images as a function of multi-frequencies (-10ppm to10ppm) for a single slice. The multi-frequency images describe smoothly varying saturation contrasts, especially yielding low SNR images around 0 ppm indicating that there exists sufficient saturation during the acquisition. Fig. 3 represents images, z-spectrum, and MTR maps. The proposed acquisition yields high quality images without apparent contrast loss, thus reflecting the reasonable z-spectrum and MTR maps to a certain extent.Conclusion
The CAIP sampling based 3D EPI was combined with spectral random walk to exploit spectral redundancy under the framework of EPI acquisition. This approach allows 9-fold acceleration with 1mm3-isotropic resolution in about 5 min scan time for whole-brain CEST source image acquisition. We successfully demonstrated the feasibility of the proposed method to generate z-spectrum and MTR map at 7 Tesla.Acknowledgements
No acknowledgement found.References
1. Guanshu Liu et al, NMR BIOMED 26: 810-828 (2013)
2. Peter C.M. van Zijl et al, MRM 65: 927-948 (2011)
3. Felix A. Breuer et al, MRM 55: 549-556 (2006)
4. Rudiger Stirnberg et al, MRM 76: 1517-1523 (2016)
5. Ricardo Otazo et al, MRM 64: 767-776
6. Mina Kim et al, MRM 61: 1441-1450 (2009)
Figures
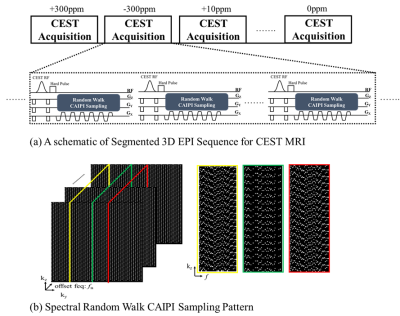
Fig1 (a) The proposed 3D segmented pulse sequence with spectral random walk sampling, (b) The CAIPI sampling is combined with spectral random walk by randomly shifting the spatial CAIPI sampling within a ky-kz block along the spectral dimension
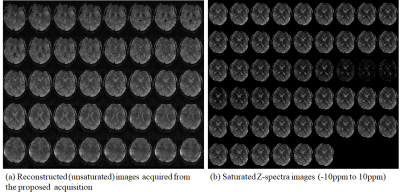
Fig2
(a) Reconstructed (unsaturated) images acquired from the proposed acquisition, (b) Saturated Z-spectra images offset frequencies -10ppm to 10ppm
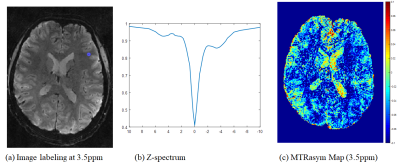
Fig3
(a) CEST labeling image at 3.5ppm, (b) Z-spectrum with marked as blue circle in
(a), and (c) MTR asymmetric map at 3.5ppm.