1457
Deep Learning-based image reconstruction improves CEST MRI
Shu Zhang1, Xinzeng Wang2, F. William Schuler1, R. Marc Lebel3, Mitsuharu Miyoshi4, Ersin Bayram2, Elena Vinogradov5, Jason Michael Johnson6, Jingfei Ma7, and Mark David Pagel1,7
1Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States, 2Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States, 3Global MR Applications & Workflow, GE Healthcare, Calgary, AB, Canada, 4Global MR Applications & Workflow, GE Healthcare Japan, Tokyo, Japan, 5Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 6Neuroradiology, MD Anderson Cancer Center, Houston, TX, United States, 7Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States
1Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States, 2Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States, 3Global MR Applications & Workflow, GE Healthcare, Calgary, AB, Canada, 4Global MR Applications & Workflow, GE Healthcare Japan, Tokyo, Japan, 5Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 6Neuroradiology, MD Anderson Cancer Center, Houston, TX, United States, 7Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Chemical exchange saturation transfer (CEST) measurements can be compromised by a low signal-to-noise ratio (SNR) due to the small CEST contrast in vivo. Deep learning-based image reconstruction (DL Recon) can enhance image SNR without losing image resolution or altering the image contrast, hence has the potential to improve quantitative CEST measurements. In this study, we investigated the improvement to CEST quantitation by DL Recon in glioma patients. We found that DL Recon substantially reduced the noise in the MTRasym maps and improved the lesion conspicuity.
Introduction
In vivo chemical exchange saturation transfer (CEST) contrast is usually small, and its quantitation can be compromised by a low image signal-to-noise ratio (SNR).1 Deep learning-based image reconstruction (DL Recon) enhances image SNR without decreasing image resolution or altering the image contrast. In a previous study, we have demonstrated that DL Recon can compensate for low SNR in CEST imaging, as was quantified using phantoms and qualitatively in 3 patients.2 The SNR gain enables CEST imaging with higher spatial resolution without or with only a mild increase in scan time. Alternatively, the SNR gain by DL Recon allows faster CEST acquisition through parallel imaging with a higher acceleration factor. In this study, we investigated the improvements of CEST quantitation by DL Recon in 10 glioma patients.Methods
Ten patients were enrolled in this IRB-approved study. 8 patients were scanned on a 3T human scanner (GE Healthcare, Discovery 750) using an 8-channel head coil. 2 patients were scanner on a PET/MR human scanner (GE Healthcare, Signa) using an 8-channel head coil. 29 CEST images with saturation from -7 to 7 ppm with equal intervals were acquired using a saturation power of 2.0 μT and a saturation time of 2 sec.3 A reference image was acquired without CEST saturation. 11 WASSR images from -1.88 to 1.88 ppm with equal intervals were acquired in the same scan for B0 inhomogeneity correction.4 All the images were acquired using a single-shot fast spin echo sequence with a matrix size of 256 and parallel imaging (ASSET = 2). MTRasym was calculated for CEST quantitation. In addition to the standard image reconstruction (standard recon), a vendor supplied DL Recon was used to reconstruct images using the same CEST raw data.5 The DL Recon network was based on a deep convolutional residual encoder pre-trained to reduce image noise without a loss in spatial resolution. The reconstructed CEST images were registered to the reference image to reduce motion artifacts. A tumor ROI and a background ROI containing homogeneous brain tissue were manually drawn on the reference images. The standard deviation of the MTRasym within the background ROI was used to characterize the noise level in the MTRasym maps. The DL Recon’s SNR improvements were calculated by dividing the MTRasym within the tumor ROI by the standard deviation of the background ROI. The tumor ROI-averaged MTRasym and the SNR values using the two reconstruction methods were evaluated using Wilcoxon matched-pairs signed rank test. P < 0.05 was considered statistically significant.Results
Fig. 1 compares the reference images, MTRasym maps at 3.5 ppm, and MTRasym maps averaged between 3.0-4.0 ppm using standard recon and DL Recon. The images using DL Recon had lower noise and were sharper than those using standard recon (Fig. 1a vs. 1d). The images using DL Recon generated MTRasym maps at both frequency ranges had less noise, and therefore there was greater tumor conspicuity (Fig. 1b vs. 1e and 1c vs. 1f). Fig. 2 compares the MTRasym at 3.5 ppm and averaged between 3.0-4.0 ppm within the tumor ROI using standard recon and DL Recon. The tumor ROI-averaged MTRasym at both frequency ranges using the two reconstruction methods was similar for all patients (Fig. 2a, p = 0.322; 2b, p = 0.492), indicating that DL Recon did not alter the CEST contrast. The tumor ROI-averaged MTRasym divided by the standard deviation of the background ROI showed that DL Recon had significantly improved SNR of the CEST measurements (Fig. 3a, p = 0.002; 3b, p = 0.016).Discussion and Conclusion
Our study showed that the DL Recon can reduce the noise in the CEST images and the calculated MTRasym maps, leading to better lesion conspicuity. With the SNR improvement, DL Recon can potentially improve a voxel-wise analysis within clinically feasible scan times. The results demonstrated that DL Recon was applicable to CEST imaging and may improve its clinical applications.Acknowledgements
This work was supported in part by the Odyssey Program and Cockrell Foundation Award for Scientific Achievement at The University of Texas MD Anderson Cancer Center (S.Z.).References
- Zhou J, Blakeley JO, Hua J, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008; 60(4): 842-849.
- Zhang S, Wang X, Schuler FW, et al. Deep Learning Reconstruction improves CEST MRI. Proc. Of ISMRM 2020, 3100.
- Miyoshi M, Matsuda T, Kabasawa H. CEST imaging with phase cycled rectangular RF preparation pulse: analytical solution, simulation and phantom study. Proceedings of ISMRM 2014, 3299.
- Kim M, Gillen J, Landman BA, et al. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009; 61(6): 1441-1450.
- Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:2008.06559 2020.
Figures
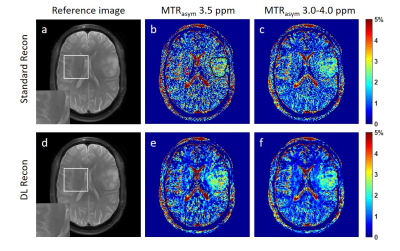
Figure 1. The reference images using standard
recon (a) and DL Recon (d). The MTRasym map at 3.5 ppm (b,e) and
averaged between 3.0-4.0 ppm (c,f) using standard recon (b,c) and DL recon
(e,f) of a glioma patient. The tumor ROI (black) and the background ROI of
homogenous brain tissue (red) was shown in (a) as an example.
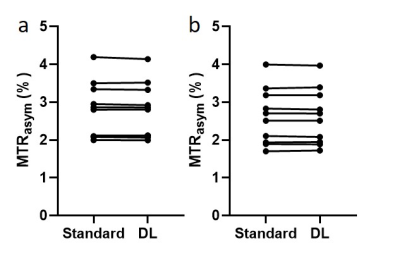
Figure 2. The MTRasym at 3.5 ppm (a) and averaged between 3.0-4.0 ppm
(b) within the tumor ROI (red in Fig. 1a as an example) using standard recon
and DL Recon was compared for all patients. (a) p = 0.322. (b) p = 0.492.
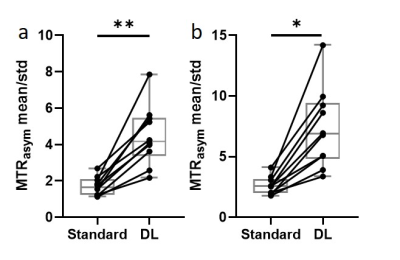
Figure 3. The tumor ROI-averaged MTRasym divided by the standard
deviation of the background ROI at 3.5 ppm (a) and 3.0-4.0 ppm (b) using
standard recon and DL Recon were compared for all patients. (a) p = 0.002. (b)
p = 0.016. **: p < 0.01. *: p < 0.05.