1456
Whole-Brain Steady-State CEST at 3T Using MR Multitasking1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, UCLA, Los Angeles, CA, United States
Synopsis
We propose a fast 3D steady-state CEST (ss-CEST) method at 3T using MR Multitasking. By exploiting the correlation among images throughout the spatial, time, and offset frequency dimensions, the low-rank tensor framework shows a possibility for at least 2x acceleration of ss- CEST. High-quality quantitative maps can be generated from ss-CEST images. The Z-spectrum acquisition with whole-brain coverage can be done within 5.5min.
Introduction
Chemical exchange saturation transfer (CEST) is a non-contrast imaging technique that indirectly detects exchangeable protons in the water pool by pre-saturation at different frequency offsets. A dense and wide sampling of the Z-spectrum is often needed for multi-pool analysis1. To ensure a clinically acceptable total scan time, the acquisition duration per frequency offset must be in seconds, including the long pre-saturation module. Therefore, it often allows only single-shot k-space acquisition, making 3D CEST challenging. One approach is to optimize the sampling efficiency, which was the focus of the recent snapshot-CEST method2-4. Another approach, steady-state CEST (ss-CEST), is to perform pre-saturation and k-space sampling in an interleaved way with repeated modules5,6. However, it requires more than 12 min to acquire the whole Z-spectrum, which is still too long for practical use. In this work, we propose a new 3D ss-CEST method using MR Multitasking7. With low-rank tensor modeling, the correlation among images acquired at different offset frequencies is exploited to further reduce the scan time and to enhance image quality.Methods
Image model:5D images were modeled as a 3-way low-rank tensor $$$\mathcal{A}$$$ with dimensions of voxel index $$$\bf{x}$$$, pre-saturation frequency offset $$$z$$$, time to reach steady-state $$$\tau$$$:
$$\mathcal{A}=\mathcal{G}\times_1{\bf{U_x}}\times_2\textbf{U}_{z}\times_3\textbf{U}_{\tau}\qquad\text{(1)}$$
where columns of each $$$\bf{U_x}$$$, $$${\textbf U}_z$$$ and $$${\textbf U}_{\tau}$$$ contain the basis functions for spatial, offset frequency, and time to reach steady-state, respectively, and $$$\mathcal{G}$$$ denotes the core tensor. The core tensor $$$\mathcal{G}$$$ and non-spatial basis matrices $$${\textbf U}_z$$$ and $$${\textbf U}_{\tau}$$$ were first recovered from frequently sampled training data, and the spatial bases $$$\bf{U_x}$$$ were then reconstructed by fitting $$$\textbf{U}_z$$$ and $$$\textbf{U}_{\tau}$$$ to the remaining imaging data. Last 3D images of each offset frequency, i.e. $$$\tilde{\bf{A}}(\textbf{x},z)=\textbf{A}(\textbf{x},z,\tau_\max)$$$, were extracted to represent the Z-spectrum images at steady-state for CEST analysis.
Data acquisition:
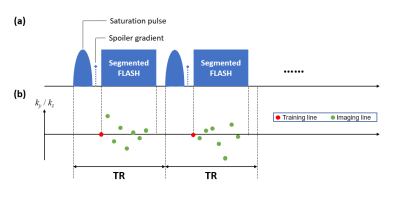
Data were acquired in five (n=5) healthy volunteers on a 3T system (MAGNETOM Vida, Siemens Healthcare) with a 1Tx/16Rx-channel head/neck coil. Fig. 1 illustrates the proposed pulse sequence and k-space sampling pattern. Each ss-CEST module contains a single-lobe Gaussian saturation pulse (tsat=30ms, flip angle=500°), followed by a spoiler gradient and eight 5° FLASH readouts. Other parameters were: FOV=220x220x120mm3, matrix size=128x128x40, spatial resolution=1.7x1.7x3.0mm3. The acquisition time was 5.6s for each frequency. Images of 53 frequency offsets (from -100 to 100ppm) were acquired, with a prolonged unsaturated acquisition (S0) at the beginning. The total imaging time was 5.5min.
Single-slice single-shot FLASH CEST images were acquired as a reference. The Z-spectrum was sampled as in ss-CEST. Other parameters were: slice thickness=10mm, TR/FA=3000ms/5°, averages=2 . For pre-saturation, a train of 30 Gaussian pulses of tsat=30ms (duty cycle=50%) and flip angle=500° were used. The total imaging time was 5min54s. T1w images were also acquired for gray/white matter (GM/WM) segmentation.
CEST analysis:
Four-pool (NOE, APT, MT, and DS) Lorentzian fitting1 using a probabilistic model8 was employed for CEST quantification. For each pool, the probability is assumed to follow Lorentzian distributions:
$$L_i(x,A_i,W_i,C_i)=A_i\cdot\frac{W_i^2/4}{W_i^2/4 + (x-C_i)^2}\qquad\text{(2)}$$
Then, the Z-spectrum is described as
$$F(x)=1–P(\text{DS}\cup\text{MT}\cup\text{NOE}\cup\text{APT})\qquad\text{(3)}$$
Fitted CEST maps were compared within GM and WM regions among different subjects.
Results
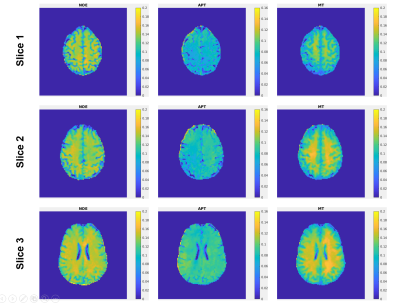
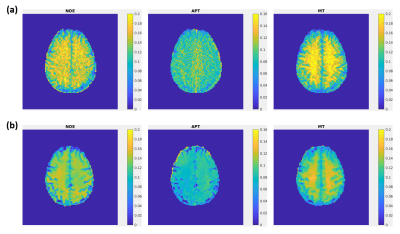
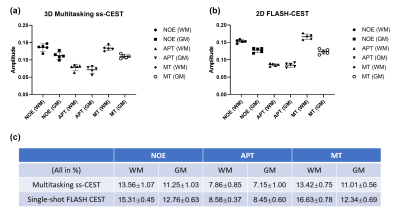
Fig. 2 shows representative NOE, APT and MT maps of three different slices fitted from the proposed method. The image qualify is consistent among different slices, except for edge slices at the boundary of the 3D volume. Fig. 3 shows the comparison between maps generated from the proposed method and the 2D single-shot FLASH method. It can be clearly seen that, while consistent with each other, the maps from Multitasking ss-CEST are less noisy. Fig. 4 shows the statistics of mean Lorentzian amplitudes within GM and WM regions among different volunteers. It can be seen that the mean amplitude is consistent among healthy subjects. The contrast ratios of WM/GM for NOE, APT, MT are 1.21, 1.10, 1.22 for Multitasking ss-CEST, which are similar to 1.20, 1,02, 1.35 for 2D single-shot FLASH CEST.Discussion and conclusion
The steady-state CEST method not only allows more k-space acquisition windows than the single-shot method, but also ensures that the steady-state is maintained during the acquisition. The Multitasking based ss-CEST has several crucial advantages by reconstructing the data from different offset frequencies all together in one tensor model: (1) further reduction of imaging time is made possible over the Nyquist criterion and limited parallel imaging factor; (2) the approach to reach steady-state within each offset frequency is modeled so that uncorrupted steady-state values will be used for quantification; (3) the Z-spectra are automatically denoised with the low-rank constraint. It also has the potential to resolve head motion within the reconstruction process9. Potential future improvements include optimization of readout trajectories (e.g., from Cartesian to spiral), further reduction of total scan time, and improving the spatial resolution.In conclusion, the proposed 3D steady-state CEST method using MR Multitasking significantly reduces acquisition time compared with traditional methods. High-quality NOE and APT maps can be generated from the proposed method.
Acknowledgements
N/A.References
1. Zaiss M, Schmitt B, Bachert P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. Journal of magnetic resonance. 2011 Aug 1;211(2):149-55.
2. Zaiss M, Ehses P, Scheffler K. Snapshot‐CEST: optimizing spiral‐centric‐reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR in Biomedicine. 2018 Apr;31(4):e3879.
3. Mueller S, Stirnberg R, Akbey S, Ehses P, Scheffler K, Stöcker T, Zaiss M. Whole brain snapshot CEST at 3T using 3D-EPI: Aiming for speed, volume, and homogeneity. Magnetic Resonance in Medicine. 2020 May 9.
4. Deshmane A, Zaiss M, Lindig T, Herz K, Schuppert M, Gandhi C, Bender B, Ernemann U, Scheffler K. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magnetic resonance in medicine. 2019 Apr;81(4):2412-23.
5. Jones CK, Polders D, Hua J, Zhu H, Hoogduin HJ, Zhou J, Luijten P, Van Zijl PC. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magnetic resonance in medicine. 2012 Jun;67(6):1579-89.
6. Heo HY, Jones CK, Hua J, Yadav N, Agarwal S, Zhou J, van Zijl PC, Pillai JJ. Whole-brain amide proton transfer (APT) and nuclear Overhauser enhancement (NOE) imaging in glioma patients using low-power steady-state pulsed chemical exchange saturation transfer (CEST) imaging at 7T. Journal of Magnetic Resonance Imaging. 2016 Jul;44(1):41-50.
7. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature Biomedical Engineering. 2018 Apr;2(4):215-226.
8. Haris M, Singh A, Cai K, Kogan F, McGarvey J, DeBrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature medicine. 2014 Feb;20(2):209-14.
9. Ma S, Christodoulou AG, Wang N, Kaisey M, Sicotte NL, Li D. Motion-Resolved, 3D Whole-Brain Simultaneous T1, T2, and T1ρ Mapping using Multitasking with Application to Multiple Sclerosis: A Pilot Study. In Proceedings of the 28th Annual Meeting of ISMRM 2020 (p. 0878).
Figures