1455
In-Vivo Sub-Minute rNOE Mapping Using AutoCEST: a Machine-Learning Approach for CEST/MT Protocol Invention and Quantitative Reconstruction1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Physics, Harvard University, Cambridge, MA, United States, 3Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 4Department of Neuroradiology, University Clinic Erlangen, Erlangen, Germany, 5Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
The long acquisition-time and the semi-quantitative nature of the typical CEST-MRI experiment constitute a major obstacle for its clinical adoption. Recently, a machine-learning approach termed AutoCEST was developed, for the automatic design of the optimal acquisition schedule and the reconstruction of quantitative 2-pool CEST maps. Here, we expand this approach for in-vivo scenarios, by incorporating the semisolid-pool into the underlying computational-graph and allowing 3 pools. AutoCEST was evaluated for quantitative rNOE mapping using a GBM mouse model, resulting in a total acquisition and reconstruction times of 49.15s. The tumor rNOE volume-fraction was significantly decreased, in agreement with previous human studies.
Introduction
Chemical exchange saturation transfer (CEST) is a molecular imaging approach, capable of amplifying and detecting the signals associated with milli-molar concentrations of biologically interesting proteins and metabolites.1 Nevertheless, a few inherent challenges hinder its wide adoption in the clinic. (1) The CEST signal is highly dependent on the acquisition schedule properties, which challenges the comparison of finding from different groups and scanner vendors. (2) Several confounding mechanisms may bias the observed CEST signal, including the water relaxation times and the contributions from other proteins/semi-solid pools.2 (3) The typical acquisition time is long. The development of a fully-quantitative and rapid CEST technique, could provide better exploitation of the molecular information available by this contrast mechanism and would constitute a significant step toward reproducible clinical translation.Recently, a new paradigm, termed AutoCEST,3 was suggested for conducting and analyzing 2-pool CEST experiments; an MR physics governed AI system was designed to get a broadly defined clinical-scenario as input, and simultaneously output the corresponding optimal acquisition-schedule and the means to obtain quantitative reconstruction.
The purpose of this work is to expand AutoCEST for practical in-vivo imaging. By incorporating the semi-solid macro-molecule pool into the underlying computational-graph, we aimed to enable the imaging of 3 pool scenarios (compatible with any CEST compound). Not less important, a clinically relevant and challenging acquisition-time upper-limit was enforced. The method was evaluated using a GBM mouse model. As the molecular-based signal of interest, we chose the relayed nuclear Overhauser effect (rNOE) aliphatic proton pool, as it has shown potential for providing diagnostically interesting information in several clinical scenarios, including Alzheimer’s disease,4 and cancer.2
Methods
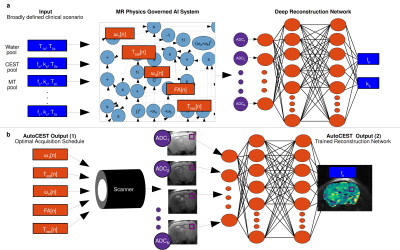
AutoCEST architectureAn overview of the AutoCEST approach is described in Fig. 1. For each scenario of interest (e.g., rNOE brain imaging, creatine muscle imaging, etc.) a pre-experiment step needs to be performed. During this step (Fig. 1a), the system gets as input a general description of the expected parameter range (Fig. 1a, blue rectangles). A random subset of parameter combinations are then input to an MR physics governed AI system, that calculates the corresponding MR signals as a result of applying a random CEST acquisition protocol. To allow optimization of the acquisition schedule parameters (orange rectangles, Fig. 1a), the CEST saturation-block is represented as a computational-graph3 based on the analytical solution of the Bloch-McConnell equations in the presence of MT.5 Next, the spin dynamics are calculated during excitation and relaxation, using the Bloch equations with a discrete-time state-space model in the rotating frame.6 This allows for the calculation of the expected “ADC” signals. Finally, the resulting MR signals are mapped to CEST/MT quantitative parameters using a fully connected 4-layers deep reconstruction network.7
In the experiment step (Fig. 1b), the optimal acquisition schedule parameters are loaded into the MR scanner, resulting in a set of N raw images. The resulting images are then fed voxel-wise into the trained reconstruction network, resulting in quantitative CEST/MT maps of the imaged subject.
Simulation studies
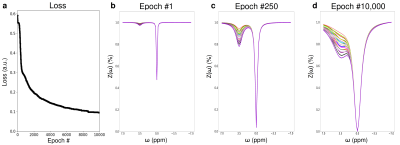
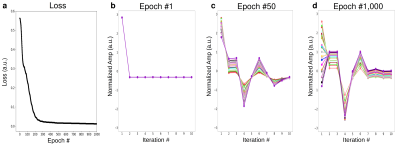
To provide intuition on the optimization procedure performed by AutoCEST, two simulations were conducted, examining an intuitive 2-pool amide imaging scenario at 9.4T. A set of 21 combinations of proton volume-fractions and exchange-rates were simulated, spanning the range of fb = 0.15-0.45% and kb = 20-40Hz. In the first simulation, AutoCEST was set to optimize the saturation-power of a standard Z-spectra, with a saturation-time (Tsat) = 5s and a repetition time (TR) = 20s. In the second simulation, an amide proton acquisition schedule is optimized in which only 10 images (iterations) were allowed, the TR was shortened to 4s, the Tsat to 2.5s, and the saturation-power was allowed to vary for each iteration, while the saturation frequency offset was fixed at 3.5 ppm.
In vivo study
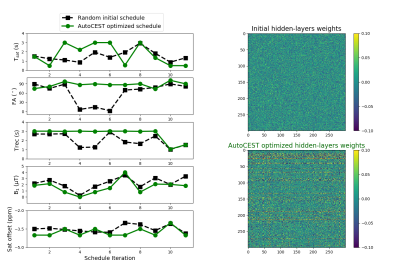
All animal procedures were approved by the institutional committee. A brain tumor (GBM) bearing mouse was imaged using a 7T preclinical MRI (Bruker, Germany). An in-house programmed flexible CEST-EPI protocol was employed, loaded with the acquisition parameters output by AutoCEST (trained for an rNOE in the presence of MT scenario).
Results
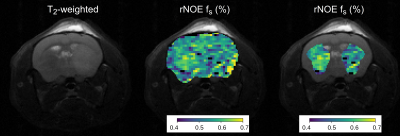
The Z-spectra optimization demonstrated a consistently improved discrimination ability for the various parameter combinations (Fig. 2). Similarly, the CEST amide schedule with the fixed saturation pulse frequency offset showed improved discrimination of different parameter combinations as the saturation pulse power was allowed to vary for different iterations, with a drastic reduction in the mean square error loss and fast convergence to the optimal solution (Fig. 3). This finding is in agreement with a previous report, showing that a varied saturation power acquisition schedule has a better parameter discrimination potential compared to a standard Z-spectra.8 The optimized in-vivo rNOE acquisition schedule is presented in Fig. 4. The reconstructed map of rNOE proton volume fraction (Fig. 5) in the tumor ROI was significantly lower compared to the contralateral ROI (two tailed t-test, p<0.001), in agreement with previous human studies demonstrating a reduced tumor rNOE CEST signal.2 The resulting AutoCEST sequence acquisition and reconstruction times were 49 s and 15 ms, respectively.Conclusion
AutoCEST was expanded to account for the MT pool, and is now suitable for in-vivo scenarios, potentially providing a fast and automatic means for designing and analyzing quantitative CEST experiments.Acknowledgements
National Institutes of Health Grant/Award Numbers: R01CA203873, P41-RR14075. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No 836752 (OncoViroMRI). This abstract reflects only the author’s view and the Research Executive Agency of the European Commission is not responsible for any use that may be made of the information it contains. This research was supported by a CERN openlab cloud computing grant.References
1) Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). Journal of magnetic resonance, 2000;143(1): 79-87.
2) Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7 T: unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 2015;112: 180-188.
3) Perlman, O, Zhu B, Zaiss M, Rosen MS, Farrar, CT AutoCEST: a Machine-Learning Approach for Optimal CEST-MRI Experiment Design and Quantitative Mapping. In 2020 ISMRM & SMRT Virtual Conference & Exhibition.
4) Chen L. et al. Protein aggregation linked to Alzheimer’s disease revealed by saturation transfer MRI. Neuroimage 2019;188:380–390.
5) Zaiss M, Zu Z, Xu J, et al. A combined analytical solution for chemical exchange saturation transfer and semi‐solid magnetization transfer. NMR in Biomedicine 2015;28(2):217-230.
6) Zhu B, Liu J, Koonjoo N, Rosen B, Rosen MS. AUTOmated pulse SEQuence generation (AUTOSEQ) and neural network decoding for fast quantitative MR parameter measurement using continuous and simultaneous RF transmit and receive. in ISMRM Annual Meeting & Exhibition, 1090. Montreal, QC, Canada: International Society for Magnetic Resonance in Medicine; 2019.
7) Cohen O, Zhu B, Rosen MS. MR fingerprinting deep reconstruction network (DRONE). Magn Reson Med. 2018;80(3):885–894.
8) Cohen, O, Huang S, McMahon MT, Rosen MS, Farrar, CT. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn Reson Med. 2018;80(6):2449–2463.
Figures