1449
Systematic characterization of MRI near a surgical breast implant at 1.5T and 3.0T1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Metallic clip soft tissue markers are a promising alternative to wire-guided localization in localizing breast cancer for surgery. However, the metallic core in the marker creates magnetic field distortions with MRI, which leads to various artifacts including signal voids and failed chemical shift-based fat suppression. This work characterizes the effect of a soft tissue marker at 1.5T and 3.0T using conventional breast imaging sequences as well as multi-spectral sequences. Several fat suppression methods were also evaluated. Field distortions and imaging artifacts are similar at 1.5T and 3.0T, and can be addressed using multi-spectral methods and T1 based fat suppression.
Introduction
Breast conserving surgery is as effective as radical mastectomy for non-palpable breast cancers[1]. Unfortunately, wire-guided localization, the most widely used method for localization of non-palpable breast lesions, has substantial drawbacks. These drawbacks include patient discomfort, the need for placement on the day of surgery, reduced surgical approaches, and the risk of dislodgement.Soft tissue markers have been developed to overcome these limitations. Recently developed markers include a metal core for localization, can be placed many days in advance of surgery and are unlikely to be dislodged[2]. Soft tissue markers are typically implanted during biopsy of suspected lesions identified using mammography or ultrasound.
Importantly, some patients may require MRI after marker placement, and the metal core within the marker can lead to MRI artifacts that degrade the utility of the acquired images. The artifacts are dependent upon the type of MRI sequence, as well as the parameters of the acquisition. However, the ability to image near these markers has not been characterized. Therefore, the purpose of this work is to evaluate a soft tissue marker localization device across several different types of MR sequences on both 1.5T and 3.0T MR systems.
Methods
Phantom ConstructionWe imaged a metallic clip soft tissue marker (SmartClip™, Elucent Medical, Eden Prairie, MN) recently developed for breast lesion localization. This marker has outer dimensions 1.4mm × 8.0mm and contains a ferrite core needed for RF-based localization. In this work, a phantom was constructed in an 11.5cm × 10cm × 8cm rectangular plastic tub using 2% weight by volume agar gel doped with 4mM copper sulfate with the soft tissue marker suspended near the center of the tub using fishing filament.
Imaging
This phantom was imaged at 1.5T (Optima MR450W and Signa Artist, GE Healthcare) and 3.0T (Signa PET/MR, GE Healthcare), with routine breast protocol sequences as well as multi-spectral imaging (MAVRIC SL) designed for imaging near metal [3]. Further, the marker was imaged while oriented both along the magnet bore (z-axis) and in an orthogonal position.
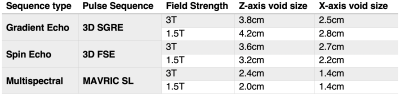
Given the importance of fat suppression in breast MRI, a 2.5cm wide by 4.5cm tall vial of peanut oil was inserted into the phantom (Figure 1), and several fat-suppression methods were also evaluated. Specific parameters for these acquisitions are shown in Table 1.
Data Analysis
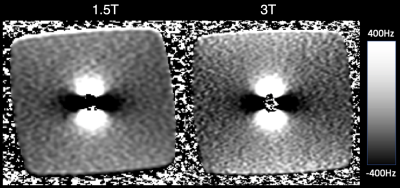
Image voids due to off-resonance introduced by the metal within the marker were measured for each type of acquisition at each field strength. MAVRIC SL was used to map the magnetic field close to the marker [4][5]. Specifically, the profile of each pixel across MAVRIC SL spectral slabs and its location in the volume was used to measure B0-induced off-resonance up to multiple kHz. This field map was fit to a point susceptibility source in order to measure the magnetization of the marker’s metallic core at 1.5T and 3.0T.
Results
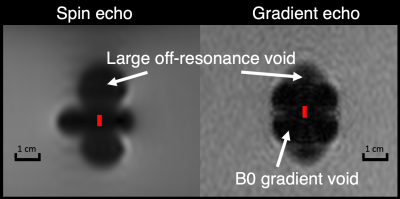
All pulse sequences suffered from signal voids near the soft tissue marker, however the shape of the void varied between spin echo and gradient echo sequences. Voids in both types of images were affected by the magnitude of the off-resonance, but gradient echo image voids were also affected by rapid spatial variation (high gradient) of the off-resonance as shown in Figure 2.No difference in void size or appearance was observed between marker orientations (parallel or perpendicular to B0, images not shown for brevity), likely due to the size of the marker relative to the image void. The image void size was nearly identical across field strengths when imaging parameters were held constant (Figure 3), suggesting no change in magnetization of the ferrite core (ie: saturation) or in the induced off-resonance. This result is also supported by the MAVRIC SL-based B0 field map (Figure 4).
When fit to a point susceptibility source, we calculated a magnetization value for the metallic core of 1.14x10-4 A×m2 at 3T, and 1.28x10-4 A×m2 at 1.5T (ie: similar values across field strengths), suggesting that the magnetization saturates below 1.5T.
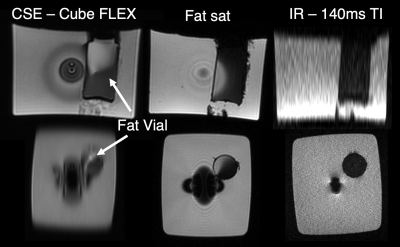
Fat suppression results are shown in Figure 5. Chemical shift-based water fat separation and fat saturation pulses failed due to the large field map perturbations produced by the metallic core. IR MAVRIC SL was able to suppress the fat, although this method led to lower SNR and a relatively long scan time (~7 minutes).
Discussion
This work evaluated the performance of conventional as well as multi-spectral MRI sequences in the presence of a soft tissue marker for breast cancer localization. A custom agar phantom was constructed to enable highly controlled experiments. As demonstrated by our results, the ferrite core of the marker presents substantial challenges to imaging with standard MRI pulse sequences. Substantial signal voids inhibit the ability to image near the marker at 1.5T and 3.0T. This challenge can be addressed in part using multi-spectral methods. Further, the field perturbations produced by the marker make fat suppression difficult using chemical shift-based methods[6]. In combination with multi-spectral imaging, inversion recovery-based fat suppression is effective, although this approach leads to SNR penalties.Acknowledgements
The authors wish to thank Elucent Medical for providing funding for this study. In addition, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. We would like to thank Dr. Suryanarayanan Kaushik from GE Healthcare for assistance with the reconstruction of MAVRIC SL data. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. Scott Reeder has ownership interest in Elucent.References
[1] U. Veronesi et al., “Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer,” N. Engl. J. Med., vol. 347, no. 16, pp. 1227–1232, Oct. 2002, doi: 10.1056/NEJMoa020989.
[2] E. Cheang, R. Ha, C. M. Thornton, and V. L. Mango, “Innovations in image-guided preoperative breast lesion localization,” Br. J. Radiol., vol. 91, no. 1085, May 2018, doi: 10.1259/bjr.20170740.
[3] K. M. Koch et al., “Imaging near metal with a MAVRIC-SEMAC hybrid,” Magn. Reson. Med., vol. 65, no. 1, pp. 71–82, 2011, doi: 10.1002/mrm.22523.
[4] X. Shi, D. Yoon, K. M. Koch, and B. A. Hargreaves, “Metallic implant geometry and susceptibility estimation using multispectral B0 field maps,” Magn. Reson. Med., vol. 77, no. 6, pp. 2402–2413, Jun. 2017, doi: 10.1002/mrm.26313.
[5] S. S. Kaushik, C. Marszalkowski, and K. M. Koch, “External calibration of the spectral coverage for three-dimensional multispectral MRI,” Magn. Reson. Med., vol. 76, no. 5, pp. 1494–1503, 2016, doi: https://doi.org/10.1002/mrm.26065.
[6] M. R. Smith, N. S. Artz, C. Wiens, D. Hernando, and S. B. Reeder, “Characterizing the limits of MRI near metallic prostheses: Limits of MRI Near Metallic Prostheses,” Magn. Reson. Med., vol. 74, no. 6, pp. 1564–1573, Dec. 2015, doi: 10.1002/mrm.25540.
Figures