1447
Systemic effects of intrauterine contraceptive devices demonstrated by background parenchymal enhancement in DCE-MRI.1Radiology, RWTH Aachen University, Aachen, Germany, 2RWTH Aachen University, Aachen, Germany
Synopsis
According to the manufacturers intrauterine contraceptive devices (IUDs) should not be associated with systemic hormonal effects. However, women’s representatives claim that IUDs do exhibit side effects that are similar to oral or percutaneous hormonal medication. Background parenchymal enhancement (BPE) reduces the sensitivity and specificity of breast MRI and is influenced by exogenous hormonal exposures. We investigated whether IUD use is associated with BPE on contrast-enhanced breast MRI, as a surrogate marker for hormonal stimulation. We found that the application of an IUD is associated with increased BPE in breast MRI. This suggests that IUDs do have a systemic hormonal effect.
Purpose:
Levonorgestrel-releasing intrauterine contraceptive devices (LNG-IUD) are the most common method of contraception worldwide.1 Based on the manufacturers information, LNG-IUDs should not be associated with systemic hormonal effects. However, women’s representatives claim that IUDs do exhibit side effects that are similar to oral or percutaneous hormonal medication.1,2 Background parenchymal enhancement (BPE) reduces the sensitivity and specificity of breast MRI and is influenced by endo- and exogenous hormonal exposures.3,4,5 Therefore, we investigated how BPE is affected by hormonal intrauterine devices.Material and Method:
Retrospective study on 48 premenopausal women (mean age 45 years) who underwent DCE-MRI according to a standardized protocol for clinical indications with and without an IUD in place; median time between studies was 27 months (range 1–77 months); half of the women (23/48) had their first scan done with IUD, and underwent follow/up MRI after removal, the other half (25/48) had their first MRI done without IUD, and had their follow-up MRI done after IUD placement. Different radiologists assessed the degree of BPE visually according to the ACR categories between 1 and 4. In addition, BPE was quantified by ROI-based measurement, with the ROI placed in the region that appeared to exhibit strongest enhancement of the normal fibroglandular tissue. Statistical analysis were performed on the ACR categories as well as on the ROI measurements using the Wilcoxon-matched pairs signed-rank test. A p-value less than 0.05 was considered statistically significant.Result:
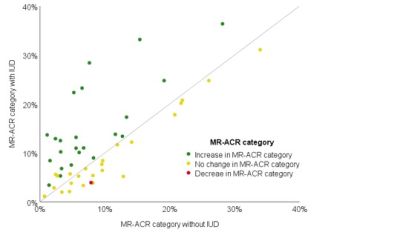
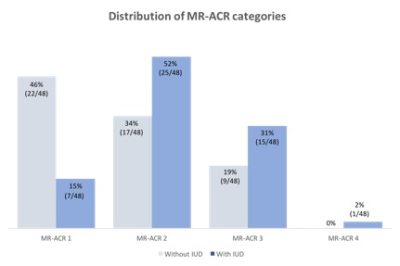
In 24/48 patients (50%; [95%-CI: 35.9%-64.1%]), ACR categories did not change with vs. without IUD. In 23/48 patients (48% [33.9%-62.1%]), the ACR category was higher with vs. without IUD; in 1/48 (2% [0%-6%]), the ACR category was lower with vs. without IUD. The change of ACR category depending on presence or absence of an IUD proved highly significant (p<0.001). A similar result was obtained for ROI-based quantitative measurement of BPE enhancement (p<0.02).Discussion:
In this intra-individual serial study of premenopausal women we investigated whether an IUD in place leads to an increase in BPE. We found a direct correlation between DCE-MRI enhancement and the presence or absence of an IUD in place. Background enhancement rates were highest in women with an IUD in place, while after removing the IUD, reduced background enhancement was observed. This is in accordance with the known fact that BPE is a biomarker for hormonal stimulation and that, against the opinion of the manufacturers, IUDs not only have an exclusively local effect in the uterus, but lead to side effects which are similar to oral or percutaneous hormonal therapies.2,6In DCE-MRI the background enhancement in breast MRI is variable and can only occur in the breasts fibroglandular component. The majority of women belong to the MR-ACR 1 category and have no enhancement of the breast tissue. The number of women with MR-ACR categories 2,3 and 4 is continuously decreasing.7 BPE is increased by hormone replacement therapy and reduced under anti-hormonal therapy, oophorectomy, tamoxifen or aromatase inhibitor treatment.8-11 All women included in this study were examined for screening or problem solving and had no history of breast cancer, of radiation therapy, of chemotherapy nor did they take any anti-hormonal therapy which effects BPE.
We hypothesize that levonorgestrel has a direct effect on the expression of factors that modulate angiogenesis and that this effect differs in every woman.12 Supporting this hypothesis, we observed that in 48% BPE was higher with an IUD in place, in 50% there was no difference in BPE and in 2% the BPE was lower with an IUD. However, we do not know the exact date of the insertion of the IUD in the uterus. Therefore, small changes in hormone levels observed by small changes in BPE could be caused by different release rates of the IUD, since the released rate of levonorgestrel decreases over time.13
For clinical practice, our data suggests that increased BPE caused by an IUD in place is common. Some studies demonstrated that strong enhancement of the normal fibroglandular tissue might mimic or obscure invasive breast cancer and DCIS which should be kept in mind in the evaluation of MRI images of women with an IUD in place.14,15
Next to mammographic breast density, the degree of BPE is a promising new marker for breast cancer risk that appears to be independent of mammographic breast density. Recent studies found an association between high levels of BPE and an increased breast cancer risk.16,17,18
Our study has limitations. We do not know the exact date of the insertion of the IUD in the uterus. Furthermore, BPE is prone to interobserver and intraobserver differences.19 By setting up an intraindividual study design of premenopausal healthy women, we tried to exclude as many confounding factors as possible.
Conclusion:
The application of an IUD leads to an increase in BPE in breast MRI which suggests that IUDs do have a systemic hormonal effect. A higher BPE hinders the differentiation of the enhancement of malignant lesions from the enhancement of normal fibroglandular tissue. This risk must be considered when evaluating breast MRI of women with an IUD in place to avoid false-positive or even false-negative diagnosis.Acknowledgements
No acknowledgements found.References
1. Nelson A, Massoudi N. New developments in intrauterine device use: focus on the US. Open Access J Contracept. 2016;7:127-141.
2. Danvers AA, Stuart GS, Bryant AG. Lawsuits against Mirena®: potential impact on public health. Contraception. 2014;89:489–492.
3. Brooks JD, Sung JS, Pike MC, et al. MRI background parenchymal enhancement, breast density and serum hormones in postmenopausal women. Int J Cancer. 2018;143(4):823-830. doi:10.1002/ijc.31370
4. King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260(1):50–60. 37.
5. Ray KM, Kerlikowske K, Lobach IV, et al. Effect of background parenchymal enhancement on breast MR imaging interpretive performance in community-based practices. Radiology 2018;286(3):822–829.
6. Reichenbach JR, Przetak C, Klinger G, Kaiser WA. Assessment of breast tissue changes on hormonal replacement therapy using MRI: a pilot study. J Comput Assist Tomogr 1999;23:407–413.
7. Hansen RK, Bissell MJ. Tissue architecture and breast cancer: the role of extracellular matrix and steroid hormones. Endocr Relat Cancer. 2000;7(2):95-113. doi:10.1677/erc.0.0070095
8. Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclicalphase dependency. Radiology 1997;203:137–144.
9. King V, Goldfarb SB, Brooks JD, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 2012;264:670–678.
10. King V, Kaplan J, Pike MC, et al. Impact of Tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J 2012;18:527–534.
11. Schrading S, Schild H, Kühr M, Kuhl C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology. 2014;271(1):45-55. doi:10.1148/radiol.13131198
12. Zeppa R. Vascular response of the breast to estrogen. J Clin Endocr 1969;29:695–700.
13. Beatty MN, Blumenthal P. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability. Ther Clin Risk Manag. 2009;5:561-574https://doi.org/10.2147/TCRM.S5624
14. Giess CS, Yeh ED, Raza S, et al: Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for falsepositiveand false-negative interpretation. Radiographics 34:234-247, 2014
15. Ray KM, Kerlikowske K, Lobach IV, et al: Effect of background parenchymal enhancement on breast MR imaging interpretive performance in community-based practices. Radiology 286:822-829, 2018
16. King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260:50–60.
17. Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology2015;276:371–80.
18. Arasu VA, Miglioretti DL, Sprague BL, et al. Population-Based Assessment of the Association Between Magnetic Resonance Imaging Background Parenchymal Enhancement and Future Primary Breast Cancer Risk. J Clin Oncol. 2019;37(12):954-963. doi:10.1200/JCO.18.00378
19. Melsaether A, McDermott M, Gupta D, et al: Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and aftertraining. AJR Am J Roentgenol 203:209-215, 2014
Figures
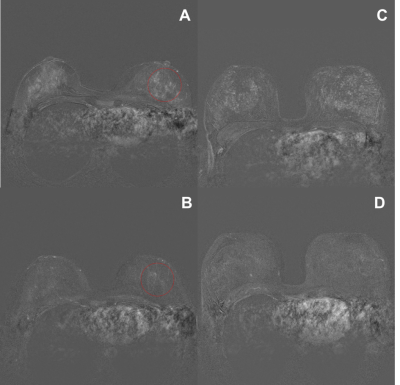
Intra-individual comparison of women with and without an IUD in place. Images show an increase in BPE with an inlaid IUD. These images show one slice of the first postcontrast subtracted images of the dynamic series.
a and b: example of ROI measurement (a) with IUD (b) without IUD
c and d: with and without an IUD in place of the same women