1442
Deep learning reconstruction including de-streaking capability for motion-robust T1-weighted breast imaging
Ping N Wang1, Sagar Mandava2, Xinzeng Wang3, Ty A Cashen4, Frederick Felcz5, and James H Holmes5
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 3Global MR Applications and Workflow, GE Healthcare, Houston, TX, United States, 4Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States, 5Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 3Global MR Applications and Workflow, GE Healthcare, Houston, TX, United States, 4Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States, 5Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
DCE imaging is the primary technique for MR evaluation of breast cancer. A key problem is ghosting due to cardiac motion obscuring axillary breast tissue. Addressing this challenge, motion-insensitive technologies such as stack-of-stars acquisition have been proposed, which introduces its own problem of streaking. Based on success with a DL reconstruction to reduce noise, blurring, and ringing, this work investigated re-purposing this deep CNN to also mitigate streaking. Phantom imaging demonstrated improved CNR and more accurate line profiles. 15 patients undergoing a clinical MR exam were scanned with the additional motion-robust method, and images showed reduced structured/unstructured noise and blurring.
Introduction
T1w 3D dynamic contrast-enhanced (DCE) MRI is widely acknowledged for having the highest sensitivity of any modality for the detection of breast cancer1–3. Breast DCE imaging is typically performed using a conventional Cartesian acquisition although radial acquisition has shown potential for breast imaging due to the ability to accelerate via angular undersampling as well as reduce sensitivity to cardiac motion4–6. However, radial sampling is known to produce streak artifacts in many settings including angular undersampling and motion. Recent developments in deep convolutional neural networks have provided substantial improvements in SNR and perceived spatial resolution7–11 In this work, we demonstrate the feasibility of combining radial acquisition with a deep learning (DL)-based reconstruction for T1w breast imaging.Methods
Human imaging was performed using a 16-channel breast coil (GE Healthcare, Waukesha, WI) for this IRB-approved and HIPAA-compliant study. 15 subjects were imaged using the routine clinical breast MR protocol at our institution including a multi-phase Cartesian acquisition during contrast injection (gadobenate dimeglumine, Multihance; Bracco Inc, Milan, Italy) followed by a radial acquisition on a 3T scanner (MR750w, GE Healthcare, Waukesha, WI). Radial imaging was performed using a 3D stack-of-stars golden-angle gradient echo imaging sequence with 256 radial projections collected at each z-phase encode. Acquisition parameters included: repetition time (TR) = 5.63 ms; echo time (TE) = 2.23 ms; field of view (FOV) = 34 cm; flip angle = 10; receiver bandwidth = +/-83.3 kHz; acquisition matrix = 448 x 448 x 142, acquired spatial resolution = 0.8 x 0.8 mm2 in-plane resolution and 1.6 mm through-plane. The radial FOV was oversampled by 2x for a total of 896 readout points to limit aliasing from signal outside the FOV.The non-Cartesian patient imaging protocol was also used to scan a resolution phantom at slice thicknesses of 0.4, 0.6, 1.0, and 1.4 mm and angular sampling factors of 2, 1, 0.5, 0.333, 0.25, and 0.125 relative to the Nyquist criterion (π/2 views to readout points). Line profiles were generated across the edge of the phantom, and CNR was calculated by: (signal in phantom – signal in background) / standard deviation of noise in background.
Non-Cartesian image reconstruction was performed using the standard pipeline and a derivative of a DL technique (AIR Recon DL, GE Healthcare, Waukesha, USA), in this case designed to reduce noise, improve sharpness, and reduce streak artifact. A convolutional neural network was constructed with ~4.4 million parameters in ~10,000 kernels. A model was trained via supervised learning which compared over 10,000 ground truth images to corresponding images degraded by noise, blurring, and angular undersampling artifact. An ADAM optimizer was used to minimize the loss between the image pairs.
Results
Images from phantom scanning are shown in Figure 1 comparing standard gridding and DL reconstructions as a function of varying slice thickness and undersampling factor. Images reconstructed with gridding (Fig. 1a) display increased noise with thinner slices and greater streaking with reduced radial sampling. However, the apparent noise and streaking are both much lower in the Radial+DL images (Fig. 1b). Note that the high frequency noise is disproportionally more suppressed in the Radial+DL images compared to the streaking artifact which was not as greatly reduced at the thinnest slices and most undersampled reconstructions. Both techniques were unable to recover image quality for the lowest Nyquist sampling factor of 0.125. From each of the images shown in Figure 1, a corresponding line profile is plotted in Figure 2. Note increased edge sharpness with the Radial+DL. Corresponding CNR measurements are shown in Figure 3 demonstrating increased CNR with the Radial+DL for all but the lowest sampling factor of 0.125. Figures 4 and 5 show two representative cases from patient scanning with visibly perceived increased SNR, reduced streak artifact, and improved overall sharpness using Radial+DL.Discussion and conclusions
Although radial acquisition mitigates the left-right ghosting artifact typically seen on breast DCE images which are due to cardiac motion and may obscure important visualization of axillary structures, any data inconsistency due to motion or overly aggressive angular understampling may lead to streak artifact instead. Still, this work demonstrates that a deep learning network can be trained to remove streak artifact, which may be less problematic than addressing Cartesian motion ghosting, even though the streak artifact is not localized. In addition, previously reported techniques to improve SNR and spatial resolution can also be incorporated into the training process. This technique may pave the way for more challenging imaging scenarios such as supine breast imaging for the purpose of surgical/radiation treatment planning or patient comfort where respiratory motion then becomes another source of artifact.Acknowledgements
The authors wish to acknowledge support from GE Healthcare, and a Research and Development Grant from the Departments of Radiology and Medical Physics, University of Wisconsin-Madison.References
- Mann RM, Cho N, Moy L. Breast MRI: State of the Art. Radiology. 2019;292(3):520–536. doi:10.1148/radiol.2019182947
- Pinker-Domenig K, Bogner W, Gruber S, Bickel H, Duffy S, Schernthaner M, Dubsky P, Pluschnig U, Rudas M, Trattnig S, et al. High resolution MRI of the breast at 3 T: which BI-RADS® descriptors are most strongly associated with the diagnosis of breast cancer? European Radiology. 2012;22(2):322–330. doi:10.1007/s00330-011-2256-6
- Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. European Radiology. 2008;18(7):1307–1318. doi:10.1007/s00330-008-0863-7
- Benkert T, Block KT, Heller S, Moccaldi M, Sodickson DK, Kim SG, Moy L. Comprehensive Dynamic Contrast-Enhanced 3D Magnetic Resonance Imaging of the Breast With Fat/Water Separation and High Spatiotemporal Resolution Using Radial Sampling, Compressed Sensing, and Parallel Imaging: Investigative Radiology. 2017;52(10):583–589. doi:10.1097/RLI.0000000000000375
- Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magnetic Resonance in Medicine. 2017;78(2):565–576. doi:https://doi.org/10.1002/mrm.26392
- Wang PN, Velikina JV, Strigel RM, Henze Bancroft LC, Samsonov AA, Cashen TA, Wang K, Kelcz F, Johnson KM, Korosec FR, Ersoz A, Holmes JH. Comparison of data-driven and general temporal constraints on compressed sensing for breast DCE MRI. Magn Reson Med. 2020 Dec 11. doi: 10.1002/mrm.28628. Epub ahead of print. PMID: 33306217.
- Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, Knoll F. Learning a variational network for reconstruction of accelerated MRI data. Magnetic Resonance in Medicine. 2018;79(6):3055–3071. doi:https://doi.org/10.1002/mrm.26977
- Malkiel I, Ahn S, Taviani V, Menini A, Wolf L, Hardy CJ. Conditional WGANs with Adaptive Gradient Balancing for Sparse MRI Reconstruction. arXiv:1905.00985 [cs, eess, stat]. 2019 May 2 [accessed 2020 Dec 16]. http://arxiv.org/abs/1905.00985
- Souza R, Lebel RM, Frayne R. A Hybrid, Dual Domain, Cascade of Convolutional Neural Networks for Magnetic Resonance Image Reconstruction. In: International Conference on Medical Imaging with Deep Learning. PMLR; 2019. p. 437–446. http://proceedings.mlr.press/v102/souza19a.html
- Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature. 2018;555(7697):487–492. doi:10.1038/nature25988
- Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. 2020 Aug 14 [accessed 2020 Dec 15]. https://arxiv.org/abs/2008.06559v1
Figures
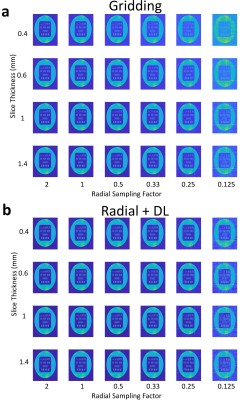
Figure 1. Phantom images are shown
for varying slice thickness (rows) and Nyquist radial sampling factor
(columns). Images reconstructed with Gridding a) and Radial+DL images b) are
displayed.
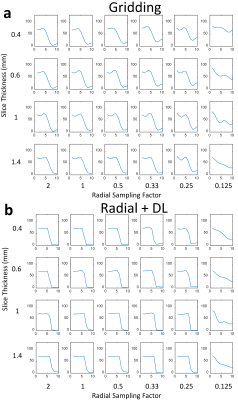
Figure 2. Line profiles depicting
the edge response in Gridding a) and Radial+DL reconstructions b). Much sharper
edge representation as well as reduced signal oscillations adjacent to the edge
are found using the Radial+DL reconstruction for Nyquist sampling factors down
to 0.25.
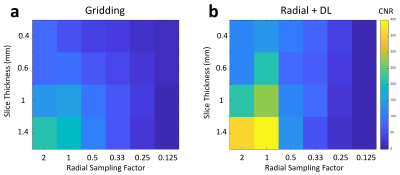
Figure 3. CNR was found to be lower
with Gridding a) compared to Radial+DL b). In general, both reconstructions
showed reduced CNR with thinner slices and a reduced Nyquist sampling factor.
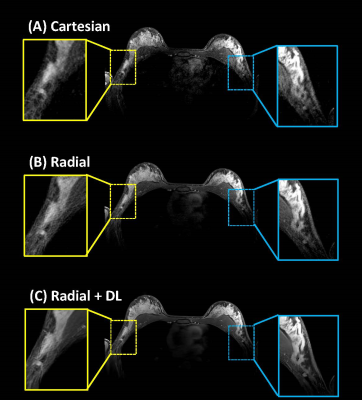
Figure 4. Bilateral contrast-enhanced
breast exam in a clinical volunteer comparing Cartesian A), Radial B), and
Radial+DL C). Enlargements of the left and right axilla show greatly improved
visual SNR with sharper edges using Radial+DL. Streak artifacts visible in
Radial (yellow box, B) is greatly reduced in the Radial+DL (yellow box, C). Note
the reduced ghosting artifact due to cardiac motion with the radial
acquisitions. Also,note improved SNR in the chest wall where coil sensitivity is
falling off.
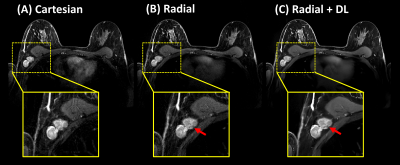
Figure 5. Bilateral contrast-enhanced
breast exam in a clinical volunteer with an enhancing mass in the axilla (red
arrows). The Radial+DL images C) showed improved SNR and image sharpness. Both
Radial reconstructions reduce aliasing due to cardiac motion but Radial+DL
shows additional reduction of streak artifacts from high contrast straight
structures such as the medial sides of the breasts. Note high contrast between
the non-enhancing structure and enhancing background of the mass (red arrows) (not
visible on the Cartesian acquisition due to out-of-plane motion).