1436
Measuring the tumour IFP using MRE: a mechanical biomarker for the prediction of metastatic potential in women with invasive breast cancer
Patriek Jurrius1,2, Omar Darwish1,3,4, Belul Shifa2, Joanna Bell2, John Spence2, Daniel Fovargue1, Giacomo Annio3, Renee Miller1, Marian Troelstra1, Ashutosh Kothari2, Hisham Hamed2, Diana Stavrou2, Ali Sever2, Sultana Hasso2, Katerina Ntailiani2, Keshthra Satchithananda5, Sarah Willson2, Sarah Pinder1,2, Anne Rigg2, David Nordsletten1, Radhouene Neji1,4, Arnie Purushotham1,2, and Ralph Sinkus1,3
1King's College London, London, United Kingdom, 2Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom, 3University Paris Diderot, Paris, France, 4Siemens Healthcare Ltd., Frimley, United Kingdom, 5King's College Hospital NHS Foundation Trust, London, United Kingdom
1King's College London, London, United Kingdom, 2Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom, 3University Paris Diderot, Paris, France, 4Siemens Healthcare Ltd., Frimley, United Kingdom, 5King's College Hospital NHS Foundation Trust, London, United Kingdom
Synopsis
Breast cancer is the most prevalent cancer among women. Over the years the overall survival has increased, with metastatic dissemination being one the most important prognostic factors. Currently, the presence of lymph node involvement or distant metastasis is diagnosed on imaging and core biopsy results. Yet, no technique to predict a cancer’s metastatic potential a priori is available. By incorporating magnetic resonance elastography (MRE) in a patient’s routine MRI scan the tumour interstitial fluid pressure, a factor associated with metastatic propensity, can be non-invasively measured. Preliminary results indicate a correlation between MRE measured tumour pressure and the cancer’s metastatic potential.
Introduction
Breast cancer is the most common cancer in women1–3. Significant progress has been made in breast cancer screening and treatment, resulting in early detection and an improved overall survival4. The 5-year survival rate is better for women with stage 1 breast cancer (97.9%) compared to late stage breast cancer, i.e. 89.6%, 72% and 26.2% for stage 2, stage 3 and stage 4, respectively5. Hence, significant emphasis is placed on treatment of the primary tumour as well as assessing and preventing metastatic spread. The intratumoural leaky blood vessels caused by the cancer-associated increased angiogenesis coupled with poor lymphatic drainage and lymphovascular invasion (LVI), results in an elevated tumour interstitial fluid pressure (TIFP) and increased tumour stiffness6–8. A high TIFP and the presence of LVI are associated with an increased risk of metastasis and thus correlate with a poor prognosis9,10. By fully integrating Magnetic resonance elastography (MRE) into a routine MRI scan, TIFP as a prognostic factor for metastatic propensity may be assessed non-invasively using nonlinear biomechanical models of tissues11. MRE is performed in addition to standard T1/T2-weighted anatomical sequences and DCE using Gadolinium12–15.Methods
Women ≥18 years of age diagnosed with invasive breast cancer scheduled to undergo primary surgery or neoadjuvant chemotherapy were recruited. MRE was carried out as part of the patient’s routine MRI, yet a breast biopsy RF-coil was used as the open design allowed for the addition of the MRE transducer, which produced mechanical waves in the breasts by driving two adjustable paddles in cranio-caudal direction16. The clinical images were obtained on a 1.5T System Area (Siemens Healthcare, Germany) using the standard-of-care MR sequences. Guided by the contrast-enhanced MR images MRE sequences were centred on the tumour core. The MRE data was acquired using the eXpresso16 sequence with 36 Hz actuation frequency17, 7 wave-phase offsets and fractional motion-encoding gradients18 of 20 mT/m. The imaging parameters of the protocol were 16 slices, 3mm isotropic voxel size, a 128 x 128 acquisition matrix, GRAPPA acceleration factor of 2, resulting in a FOV of 384 x 384 x 48 mm3, and TE = 9.2ms in-phase. The total duration of the MRE scan was 7:17 minutes.Clinical information on patient’s age, tumour size, grade, receptor status, presence of in situ disease, LVI, comedo necrosis, lymph node involvement and extranodal spread were prospectively recorded. Where available, the definitive histopathology results on the excised breast cancer and lymph node specimens were used. Alternatively, tumour characteristics and lymph node involvement were recorded from pre-treatment imaging and core biopsy results.MRE informed about the biomechanics of the tumour, providing elasticity (Gd [kPa]), viscosity (Gl [kPa]), phase angle (2/p*atan(Gl/Gd)) and TIFP [kPa]. The viscoelastic parameters were reconstructed from the displacement field by local inversion of the viscoelastic complex wave equation. A Gaussian filter was applied prior to the inversion to insure smoother spatial derivatives which were subsequently calculated in frequency space (Figure 1)19. TIFP was estimated by examining the stiffness of the surrounding tissue of the tumours (Figure 2)19. Clinical characteristics were correlated to the aforementioned MRE biomechanics parameters.Results
After excluding 5 women from analysis for various reasons, 20 women with 23 breast cancer tumours were analysed. In all, 15 tumours were grade 2, 8 were grade 3, 74% oestrogen receptor (ER) positive, 13% Her2 receptor positive and 13% triple-negative breast cancer, with an average tumour size of 28 ±22 mm. Figure 3 shows TIFP plotted for cancers without LVI nor lymph node involvement, cancers with LVI or lymph node involvement, and cancers with both LVI and lymph node involvement, representing a low, medium or high metastatic potential, respectively. This result indicates a strong correlation between MRE measured TIFP and the cancer’s metastatic potential. No correlations were found between either TIFP or the viscoelastic parameters and any of the other clinical features.Discussion
Definitive histopathology results on the excised breast cancer and axillary lymph nodes specimens were available for 9 out of the 24 tumours. For the remaining 15 tumours, the lymph node status as determined on routine imaging and the pre-treatment core biopsy results were used. Though being a reliable approximation of the patient’s clinical status, it lacks complete accuracy compared to the gold standard histopathology assessment of the surgical specimens. Therefore, the number of women found with LVI and/or involved lymph nodes is likely to be an underrepresentation. A proportion of women with a high TIFP in the absence of LVI and lymph node involvement may therefore prove to have LVI and/or lymph node involvement based on their subsequent final post-surgical histopathology results. Thus, a stronger correlation between LVI +/- lymph node involvement and MRE measured TIFP to predict metastatic potential may be expected. Although interesting trends were observed, only a small number of tumours were available for this early analysis. Investigations on a larger patient cohort are ongoing.Conclusion
Early analysis of exploratory data indicates that non-invasive measurements of TIFP by MRE correlates with LVI and/or lymph node involvement, which are prognostic factors for metastatic dissemination of breast cancer. Therefore, MRE may have the potential to be an early mechanical biomarker for the metastatic propensity of invasive breast cancer.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement 668039.References
- International Agency for Research on Cancer. GLOBOCAN 2018 - Cancer incidence and mortality statistics worldwide and by region. (2018).
- Cancer Research UK. Worldwide cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (2018).
- Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 (2010).
- Cancer Research UK. Breast cancer survival statistics | One-, five-, ten-year survival for breast cancer in England 2013-2017. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Zero (2020).
- Cancer Research UK. Breast cancer survival statistics | Breast cancer survival by stage at diagnosis - Five-year net survival by stage in England 2013-2017. 2020.
- Heldin, C. H., Rubin, K., Pietras, K. & Östman, A. High interstitial fluid pressure - An obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004).
- Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006).
- Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
- Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
- Zhang, S. et al. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: A systematic review and meta-analysis. Oncotarget 8, 2863–2873 (2017).
- Fovargue, D. et al. Towards noninvasive estimation of tumour pressure by utilising MR elastography and nonlinear biomechanical models: a simulation and phantom study. Sci. Rep. 10, 1–13 (2020).
- Muthupillai, R. et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (80-. ). 269, 1854–1857 (1995).
- Sinkus, R. et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 23, 159–65 (2005).
- Sinkus, R. et al. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn. Reson. Med. 58, 1135–44 (2007).
- Xu, L. et al. Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 48, 327–30 (2007).
- Garteiser, P. et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed. 26, 1326–1335 (2013).
- Runge, J. H. et al. A novel magnetic resonance elastography transducer concept based on a rotational eccentric mass: Preliminary experiences with the gravitational transducer. Phys. Med. Biol. 64, (2019).
- Rump, J., Klatt, D., Braun, J., Warmuth, C. & Sack, I. Fractional encoding of harmonic motions in MR elastography. Magn. Reson. Med. 57, 388–395 (2007).
- Sinkus, R. et al. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn. Reson. Med. 53, 372–387 (2005).
Figures
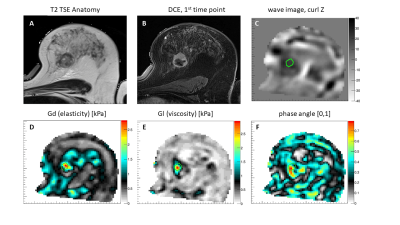
Figure 1. Results for a patient with lymph node positive involvement: T2-TSE anatomical scan (A), 1st dynamic DCE image after bolus injection (B), corresponding wave image of the MRE scan demonstrating efficient wave penetration within the entire breast (C), reconstructed images of elasticity, viscosity, and phase angle respectively (D,E,F) characterising the lesion as a stiff and very viscous mass.
Figure 2. Variation of elasticity around two selected tumour cases (left: LVI+, right: LVI- and no lymph node involvement) as a function of contraction parameter α11. Minima in the variances indicate the state at which the virtual contraction of the object removes its impact on the stiffness in the vicinity of the tumour. The value of α at the minimum is then converted to pressure (p) assuming a background stiffness of 0.5kPa. The LVI+ case exhibits an elevated pressure (p = 1.1kPa), while the LVI- and lymph node negative case is compatible with zero pressure.
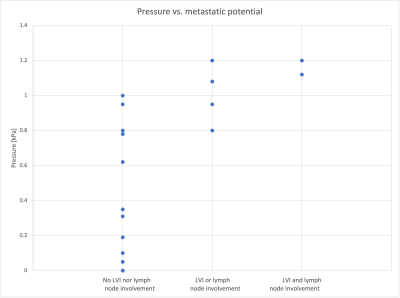
Figure 3. Distribution of tumour pressure in kilopascal (kPa) as quantified by MRE for the cancers that did not show any lymphovascular invasion (LVI) nor lymph node involvement vs. cancers with either LVI or lymph node involvement and cancers showing both LVI and lymph node involvement on histopathological and imaging assessment. This figure indicates that a higher metastatic potential, i.e. presence of LVI and/or lymph node involvement, is associated with a higher pressure.