1433
Predictive value of APTw for early evaluation of pathological complete response to NAC in molecular subtyping of breast cancer1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Dalian Medical University, Dalian, China, 3Philips Healthcare, Beijing, China, Beijing, China
Synopsis
Amide proton transfer (APT) imaging is based on the chemical exchange between free bulk water protons and the amide protons (-NH) of endogenous mobile proteins and peptides in tissue[1]. Nan Meng et al suggested that APTw imaging can be used for the differential diagnosis of benign and malignant breast lesions[2]. A simple sample study has shown that APTw MRI provides a possible biomarker for assessing chemotherapy response in human breast cancer patients on 3T MRI[3]. This study aims to explore the feasibility of APTw-MRI in early evaluation of pathological complete response to NAC in molecular subtyping of breast cancer
Introduction
APTw MRI has good potential to detect breast tumors and discriminate malignant and benign breast tumors before surgery. CEST MRI of the amide protons is potentially sensitive to microstructural molecular changes that occur prior to macroscopic changes in gross morphology and traditional contrast mechanisms.It is required for a more comprehensive analysis of APTresidual measures in relation to neoadjuvant chemotherapy(NAC).Methods
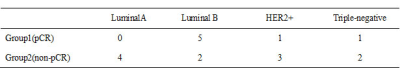
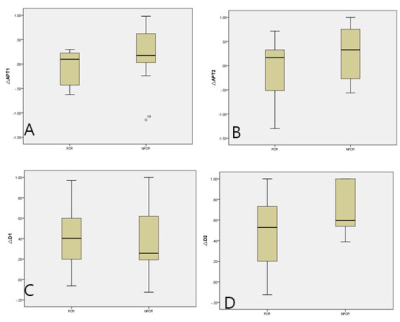
In this prospective study, a total of 18 female breast cancer patients treated with NAC (49.58±9.73, range:34-68years) were enrolled and informed consent was acquired from each patient. All patients were imaged using a 3.0 T whole-body MR scanner (Philips Ingenia CX, Philips Healthcare, the Netherlands) before NAC, at the end of the first two cycles of NAC (T1) and four cycles of NAC (T2) therapy, with a dedicated seven-channel bilateral phase-array breast coil. All patients were confirmed by surgical histopathology after MR scanning.Specifically, group 1 included 7 patients with pCR status, and group 2 included 11 patients with non-pCR status. The region of interest (ROI) was obtained manually on the APTw MR images, DCE image was used to help drawing the ROIs, which corresponding with the most enhanced part of the lesion on DCE. The mean value of ROI measurements was used as the final APTw values of lesions and maximal lesion diameter as Dmax. The measured image parameters (△APTw values, △Dmax) achieved at T1 and T2 and age were compared between two groups. Receiver operating characteristic curves (ROC) were conducted to assess the predictive capability.Results
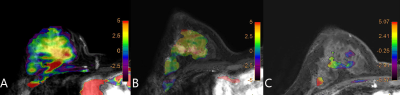
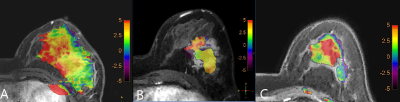
There was no significant difference in age between two groups (p =0.075). The distribution in molecular subtyping of breast cancer of two groups is in Table1.There was no significant difference of △Dmax on T1 (p =0.346), while there was significant difference between the two groups (p =0.046) on T2.The APTw maps are shown as overlays on the anatomical images with the highest correspondence in 18 lesions. The intraclass correlation coefficients (ICC=0.993, 0.991, and for group 1 and 2, respectively) indicate a good inter-observer agreement of the measured △APTw values(Figure 1). △APTw values of pCR were significantly lower than those of non-pCR status on T1 (-0.091% &0.205 %, p <0.001), while there was no significant difference of △APTw values between two groups (p =0.078) on T2(Figure 2,3). Area under the curve (AUC) acquired by △APTw MRI on T1 with an optimal threshold of 0.667 (sensitivity of 100%, a specificity of 58.3 %, and an accuracy of 41.3%).Discussion and Conclusion
Preoperative prediction for pCR is of both clinical and economic value for regimen adjustment of breast cancer, evaluation and comparison. Earlier accurate judgment for pCR status would bring larger clinical benefit for molecular subtyping of breast cancer. APT-weighted (APTw) MRI could noninvasively identify and differentiate tumors in brain, head and neck etc[4-6]. In this study, we have established the scan reproducibility of APTw imaging for observing different cycles of NAC in molecular subtyping of breast cancers, and presented initial data to show that high sensitivity performance can be achieved by △APTw values for early prediction of pCR status at the end of the first two NAC cycle, which might allow timely regimen refinement before definitive surgical treatment.Acknowledgements
This work was supported by the grant of the Provincical Natural Science Foundation of China (2019-ZD-0907, 20170540296) .References
[1] Zhou J, Heo HY, Knutsson L, et al. APT‐weighted MRI: techniques, current neuro applications, and challenging issues. Magn Reson Imaging. 2019,50(2) :347-364.
[2] Nan M,Xue W,Jing S,et al. Comparative Study of Amide Proton Transfer-Weighted Imaging and Intravoxel Incoherent Motion Imaging in Breast. J Magn Reson Imaging.2020 10 ;52(4):1175-1186
[3] Dula AN, Arlinghaus LR, Dortch RD, et al. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med 2013;70(1): 216-224.
[4] Tanoue M, Saito S, Takahashi Y, et al. Amide proton transfer imaging of glioblastoma, neuroblastoma, and breast cancer cells on a 11.7T magnetic resonance imaging system. Magn Reson Imaging 2019;62:181-190.
[5] Heo HY, Zhang Y, Jiang S,et al. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals. II: Comparison of three EMR models and application to human brain glioma at 3 Tesla. Magn Reson Med. 2016,75(4):1630–1639.
Figures