1431
Kinetic parameters derived from ultrafast DCE-MRI to predict breast cancer response to neoadjuvant chemotherapy1University of Chicago, Chicago, IL, United States, 2The University of Texas at Austin, Austin, TX, United States
Synopsis
We retrospectively reviewed data from 11 patients who received NAC and were scanned with a protocol that included ultrafast DCE-MRI for the first minute after contrast injection, followed by high spatial-resolution post-contrast imaging prior to therapy and post-treatment. The results showed a significant correlation between response to therapy and the contrast media bolus arrival time (BAT) in tumor, and normal parenchyma in each breast separately. In addition, BAT is significantly associated with background parenchymal enhancement (BPE) while BPE rate is independent of BPE. BPE rate and tumor enhancement rate may be independent predictors for malignancy.
Introduction
Standard and ultrafast dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) provide important markers for detection of breast cancer and evaluation of response to therapy.1, 2 Recent studies found an association between decreased background parenchymal enhancement (BPE) and pathologic complete response (pCR).3 However, BPE varies between individuals and is strongly influenced by hormonal milieu.3 Kinetic parameters derived from ultrafast DCE-MRI, such as initial enhancement rate and bolus arrival time (BAT) in lesions, are strongly linked to breast cancer characteristics,4,5 while those kinetic parameters for normal parenchymal tissue are rarely discussed. In this study, we investigate the relationship between ultrafast DCE-MRI parameters and the residual cancer burden (RCB) following therapy.Methods
IRB approved retrospective study waived written informed consent. Breast MRIs between Feb 2017 and Feb 2020 were retrospectively reviewed to meet following inclusion criteria: (1) two MRIs – prior to therapy and after the completion of neoadjuvant chemotherapy (NAC), (2) MRI on 3T scanner with 16-channel breast coil for all exams, (3) a hybrid clinical protocol with ultrafast DCE-MRI (temporal resolution of 3 – 7 sec) for 60 sec after contrast injection followed immediately by standard DCE-MRI for all exams. This preliminary study included 11 patients with biopsy-proven invasive ductal carcinoma (IDC) grade II (n = 3) or III (n = 8). The median patient age = 58 years (range 41 – 74). The tumor grade and residual cancer burden (RCB) class for each case were obtained from the surgical pathology reports.Semi-automatic volumetric segmentation of whole breast and parenchymal tissue was performed on the first pre-contrast image of ultrafast DCE-MRI.6 Tumors were manually segmented under the guidance of the radiologist. Voxel-based percent enhancement (PE) versus time from ultrafast DCE-MRI was fit to a truncated (uptake only) empirical mathematical model (EMM):7
$$PE(t) = A \cdot \frac{(\alpha(t-t_0))^2}{1+((\alpha(t-t_0))^2} \cdot (t>t_0)$$
where $$$A$$$ is the upper limit of percent enhancement, $$$\alpha$$$ is the uptake rate (sec-1) and $$$t_0$$$ is the bolus arrival time (BAT). We used $$$A\cdot \alpha$$$ for the initial enhancement rate.
Ultrafast DCE-MRI-derived kinetic parameters – initial enhancement rate (sec-1) in normal parenchyma and tumors, BAT in normal parenchyma (pBAT, sec) and in tumor (tBAT, sec) –were calculated for both the affected and contralateral breast parenchymal tissue. BPE was measured as the mean of percent signal enhancement across all voxels identified as normal parenchyma at the final ultrafast time-point. Tumor percent enhancement was measured by averaging across the tumor ROI at the same time-point.
Results
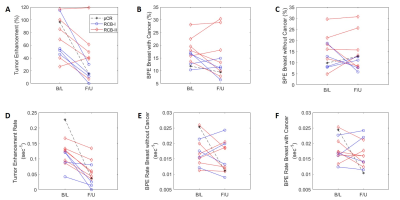
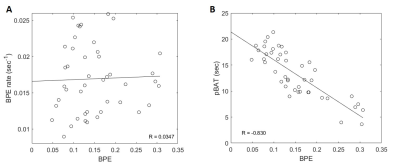
Tumor initial enhancement rate declined in all cases following NAC (Figure 1D). Tumor percent enhancement decreased in all RCB-I and pCR cases, but in 2 of the RCB-II cases this was not the trend (Figure 1A). A lower RCB class (including pCR) corresponded to lower tumor enhancement and tumor initial enhancement rate.Figure 2 compares BPE with the BPE rate and pBAT. BPE was inversely correlated with the pBAT, i.e. earlier pBAT is linked to larger BPE. No significant correlation was found between BPE and BPE rate.
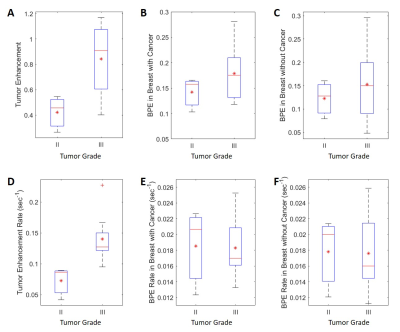
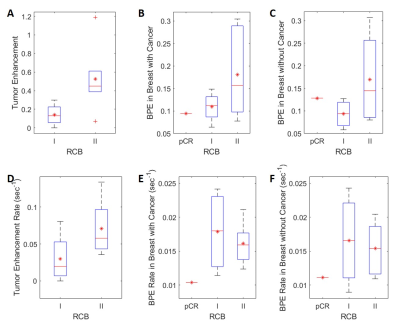
Figure 3 shows percent enhancement and enhancement rate for different tumor grades. Higher tumor grade was associated with higher tumor percent enhancement and higher tumor enhancement rate. RCB-II was associated with higher tumor percent enhancement and higher tumor enhancement rate vs. RCB-I (Figure 4). However, BPE rate is not associated with tumor grade or RCB grade.
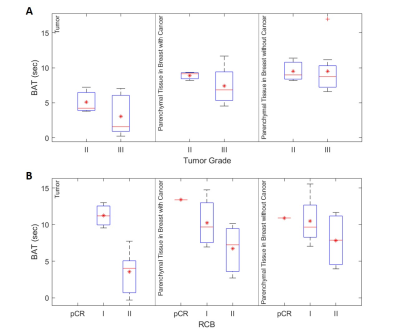
Figure 5 (A) shows BAT measured in the pre-treatment scan. The contrast bolus arrived much earlier in the tumor than in the parenchymal tissue (3.6 ± 2.7 vs 8.7 ±2.7 sec), while pBAT in the affected breast was, on average, 1.8 sec shorter than pBAT in the contralateral breast. Compared to tumor grade 2, tumor grade 3 was associated with a shorter mean tBAT and a larger pBAT difference between two breasts. Post-NAC (Figure 5 (B)), BAT values in RCB-II cases remained the same as in pre-treatment scan. However, BAT was similar (10.8 ± 2.7 sec) in tumor and bilateral parenchyma when the response was classified as RCB-I or pCR.
Discussion
Like BPE, pBAT varies significantly between individuals. However, pBAT in the affected breast is often shorter than pBAT in the contralateral breast. This suggests that angiogenic processes in cancers increase vascular permeability and blood flow in the ipsilateral ‘normal’ parenchyma. This phenomenon is more pronounced with higher tumor grade.These preliminary results suggest that BAT is a useful marker for evaluating response to therapy. Non-pCR with higher RCB grade is associated with much shorter BAT in the tumor and shorter pBAT in the affected breast compared to the contralateral breast, as is the case in the pre-treatment measurements. Lower RCB grade or pCR was associated with similar BAT values in tumor and parenchymal tissue in both breasts.
Conclusion
Ultrafast DCE-MRI can provide surrogate markers for tumor angiogenesis and for response to therapy. Specifically, bolus arrival time in tumor and parenchymal tissue could be a useful prognostic marker for treatment outcome, while enhancement rate in tumor is significantly correlated with tumor grade and RCB classification. This analysis will be performed in a larger cohort to validate our results.Acknowledgements
This study is supported in part by the National Cancer Institute of the National Institutes of Health through grants U01 CA142565 and R01 CA172801, the Segal Family Foundation, and the University of Chicago Cancer Center.References
[1] Yankeelov, T.E., et al., Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging, 2007. 25(1): p. 1-13.
[2] Abe H, Mori N, Tsuchiya K, et al. Kinetic Analysis of Benign and Malignant Breast Lesions with Ultrafast Dynamic Contrast-Enhanced MRI: Comparison With Standard Kinetic Assessment. AJR Am J Roentgenol. 2016, 207(5):1159-1166.
[3] Liao, G.J., et al., Background parenchymal enhancement on breast MRI: A comprehensive review. J Magn Reson Imaging, 2020. 51(1): p. 43-61.
[4] Pineda FD, Medved M, Wang S, Fan X, Schacht DV, Sennett C, Oto A, Newstead GM, Abe H, Karczmar GS. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Acad Radiol. 2016,23(9):1137-1144.
[5] Onishi, N., Sadinski, M., Hughes, M.C. et al. Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res 2020, 22(58)
[6] Fedorov A., Beichel R., Kalpathy-Cramer J., Finet J., Fillion-Robin J-C., Pujol S., Bauer C., Jennings D., Fennessy F., Sonka M., Buatti J., Aylward S.R., Miller J.V., Pieper S., Kikinis R. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012,30(9):1323-1341.
[7] Zhou X., et al., Comparison of DCE-MRI of murine model cancers with a low dose and high dose of contrast agent, Physica Medica, 2020 (Accepted).
Figures