1430
Robot Assisted Dynamic Ankle Joint Imaging with a Wearable 4-Channel High Impedance Coil at 1.5T MRI1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Gießen, Germany, 2Motionrad GmbH, Berlin, Germany, 3Department of Diagnostic and Interventional Radiology, Philipps-University Marburg, Marburg, Germany
Synopsis
Limitations of conventional MRI include its lack of ability to observe controlled joint motion and biomechanics in real-time. Although clinically evident, many injuries, instabilities, and dysfunctions of the musculoskeletal system are frequently not depicted on conventional static MRI. To enable dynamic MRI for joints, an in-bore motion-assisted device and a wearable coil array was designed, constructed, and validated. The combination of robotic assisted joint motion, a tight-fitting coil array that does not restrict the joint's range of motion, and accelerated imaging enabled dynamic MRI of the ankle.
Introduction
Many musculoskeletal disorders are strongly correlated to pathomechanical motion patterns. While conventional static MRI in musculoskeletal (MSK) diagnostics is widely used to assess the structure of various joints, it does not provide adequate information about their functional status and fails to detect many injuries, instabilities, and syndromes1-4.Dynamic MRI examination for quantifying functional joint motion can be found since early 90’s5,6. Commonly, the patient’s joint movements have been performed actively and unassisted. However, only controlled passive joint motion provides information about intrinsic motion pathologies7,8.
In order to unlock the full potential of dynamic joint MRI, robotic assisted joint movements and accelerated imaging with tight-fitting flexible array coils are critical. Therefore, the primary rationale of this hardware study was to develop an MRI compatible robotic device for controlled ankle movements and combine this technology with a highly flexible High Impedance Coil (HIC) array which was designed like a sock.
Methods
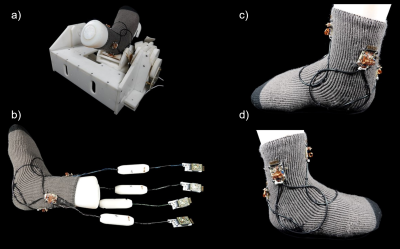
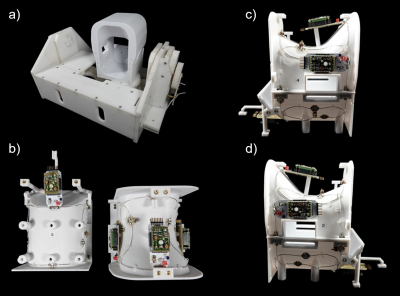
Wearable 4ch High Impedance Coil: The design of the coil elements is based on the work of Zhang et al9. The coils were made from a 50 Ohm coaxial cable (G 02232, HUBER+SUHNER, Herisau, Switzerland). The length was chosen so that the cable would have approximately the correct resonant frequency with the outer conductor shorted and with a gap in the middle of the outer conductor. The inner conductor was soldered to a small output port that also comprises the active detuning circuit, the matching network, and a trimming capacitor for fine tuning. The circular overlapped HIC loops encompassed the ankle and were sewn onto a sock (Fig.1). The circuit board was connected to the preamplifier with a coaxial cable, carefully adjusted in length, in order to transform the preamplifier’s impedance to a short at the coil terminal.Rigid 4ch Low Impedance Coil: For imaging performance evaluation, the 4ch HIC array coil was compared to a regular 4ch phased-array coil10, which was designed in the conventional manner. This Low Impedance Coil (LIC) array consists of a tight-fitting splittable housing, where the patient’s ankle can be easily placed (Fig.2). The coil housing was mounted on the robotic motion device. The 4 geometrically overlapped LIC loop elements were constructed from silver-plated copper wire and also encompassed the ankle joint. All adjacent coil elements were critically overlapped and additionally decoupled using preamplifiers. The active detuning circuitry and matching network were directly integrated into the preamplifier's daughterboard.
Robotic Motion Device: We have further developed our MR compatible robotic motion device7,8, to be compatible with the two 4ch array coils. The robotic device uses two piezo motor to perform controlled physiological movements such as dorsal extension/plantar flexion as well as pronation/supination.
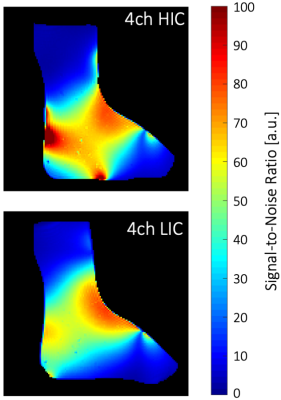
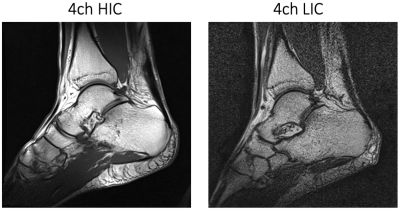
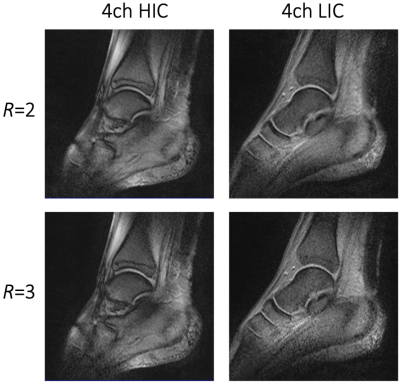
Image acquisition: Phantom and volunteer imaging was carried out on a 1.5T clinical scanner (MAGNETOM Espree, Siemens Healthineers AG, Erlangen, Germany). Pixelwise SNR calculations were derived from a PD weighted sequence (TR/TE/α=300ms/5ms/20°, matrix: 256x256, slice: 4mm, BW=150Hz/px) and followed the Kellman method11 (Fig.3). Noise correlation was obtained from the same sequence but with 0 V Tx amplitude. A single loop of HIC and LIC (diameter 12 cm) was constructed for direct SNR comparisons between the two receive coil technologies. Initial volunteer imaging compared high-resolution anatomical T1 imaging between the 4ch-HIC and 4ch-LIC arrays (Fig.4). Accelerated dynamical joint imaging was demonstrated with a turbo FLASH sequence using GRAPPA factors 2 and 3 (TE/α=/3.99ms/12°, resolution: 0.8x0.8x3mm, BW=130Hz/px RGRAPPA=2: TR=1680ms; RGRAPPA=3: TR=1220ms)(Fig.5).
Results
The piezo motors and the remote-control unit of the robotic motion device were well-shielded and did not generate any artefacts in the acquired images. Adjacent loop decoupling ranged from -14 dB to -18 dB for the HIC and from -18 dB to -20 dB for the LIC. Isolation between tuned and detuned state achieved by active detuning was better than -24 dB for both 4ch coils. Preamplifier decoupling provided another -17 dB. The noise correlation for the LIC and HIC ranged from 5% to 18% and 8% to 23%, respectively. The regular single loop LIC outperformed the size matched HIC loop by 8%. However, due to the tight-fitting form factor (wearable sock), the 4ch HIC provided a 1.3-fold better SNR when compared with the 4ch LIC array. Both coils enable dynamic acquisition with GRAPPA factor 2 and 3. However, the wearable HIC coil showed overall favorable reception sensitivity for accelerated in vivo dynamic imaging.Discussion
An MRI-compatible robotic device for controlled ankle motion and a wearable high-impedance coil were developed to improve dynamic MR imaging. The intrinsic property of low coupling between the loop elements of the HIC array is well-suited for guided joint motion. The coil maintains a tight fit in any position of the ankle, thus, providing high sensitivity during the dynamic acquisition. The advantage of such robotic assisted MR imaging method in combination with a wearable HIC enables clinicians to perform controlled passive motion of joints during imaging and could potentially detect pathologies that are occult to conventional static imaging.Conclusion
By enhancing spatio-temporal resolution with a highly flexible HIC array, dynamic robotic assisted ankle imaging was feasible. The combined and orchestrated technology of wearable coils and robotic assisted motion showed the potential to expand the utility of MR in diagnosis from the biomechanical musculoskeletal system.Acknowledgements
No acknowledgement found.References
[1] Raikin SM, Elias I, Nazarian LN: Intrasheath subluxation of the peroneal tendons. J Bone Joint Surg Am. 2008 May; 90 (5): 992-9.
[2] Emerging MRI Technologies for Imaging Musculoskeletal Disorders Under Loading Stress. Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. AHRQ Publication No. 11-EHC024-EF, (2011) www.ahrq.gov.
[3] Foot Hyperbook. An evidence-based resource for education in foot and ankle surgery. http://www.foothyperbook.com/elective/ankleInstability/ankleInstabImaging.htm.
[4] The American Orthopedic Foot & Ankle Society. AOFAS. Physicians Resource Center. http://www.aofas.org/PRC/conditions/Pages/Conditions/Ankle-Instability.aspx.
[5] Yustin DC, Rieger MR, McGuckin RS, Connelly ME. Determination of the existence of hinge movements of the temporomandibular joint during normal opening by Cine-MRI and computer digital addition. J Prosthodont Off J Am Coll Prosthodont. 1993;2: 190–195.
[6] Melchert UH, Schröder C, Brossmann J, Muhle C. Motion-triggered cine MR imaging of active joint movement. Magn Reson Imaging. 1992;10: 457–460.
[7] Elias I, Zoga AC, Schweitzer ME, Morrison WB, Abolmaali N. Controlled Dynamic Movement of Joints and Spine during Ultra-fast MRI Using a Motor Driven Device: Feasibility and Practical Application, RSNA, Annual Meeting, Chicago,USA (2011)
[8] Elias I, Abolmaali N, Morrison WB, Vaccaro AR, Lohrer H, Vogl TJ: Real Time Passive Motion of the Musculoskeletal System Using a Motor Driven Kinematic Device During Ultra Fast MRI. ISMRM Annual Meeting, Miami, USA (2005).
[9] Zhang B, Sodickson DK, Cloos MA. A high-impedance detector-array glove for magnetic resonance imaging of the hand. Nat Biomed Eng. 2018. doi: 10.1038/s41551-018-0233-y.
[10] Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16(2):192-225. doi:10.1002/mrm.1910160203
[11] Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005 Dec;54(6):1439-47. doi: 10.1002/mrm.20713. Erratum in: Magn Reson Med. 2007 Jul;58(1):211-2. PMID: 16261576
Figures