1416
Stabilization of bias field on 3D MPRAGE at 7T with dielectric pads and 3D-based B1+ scaling1Neurobiology Research Unit, Dept. of Neurology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Hvidovre, Denmark, 3Section for Magnetic Resonance, DTU Health Tech, Technical University of Denmark, Kgs. Lyngby, Denmark, 4Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, 5Philips Healhtcare, Copenhagen, Denmark
Synopsis
7T MRI has several cases of demonstrated clinical yield, but is challenged by spatial B1 inhomogeneity. Bias fields in structural images may be acceptable for visual and computational analysis, and can to some extent be accounted for by bias-field corrections. However, this is significantly complicated since the characteristics of the bias field often varies between patients. By analyzing stability of bias fields in 3D MPRAGE images, we compared two dielectric pad setups and the impact of 3D-based B1+ scaling, i.e., optimization of the RF gain. We found increased stability by using large pads and 3D-based B1+ scaling in combination.
Introduction
MRI at 7T is a promising neuroimaging tool with great potential clinical yield, e.g., in detection and delineation of brain lesions [1]. By the virtue of submillimeter spatial resolution, increased susceptibility contrast and SNR, standard structural sequences and contrast weightings may provide increased sensitivity during both visual inspection and computational methods. But 7T MRI is also challenged by increased B1+-induced spatial inhomogeneity [1,2], and 2D-based RF gain optimization may not be adequate for dealing with the large B1 field inhomogeneity variations encountered at ultra-high field. Sub-optimal RF gain optimization may cause additional B1+ inhomogeneity, which may hamper visual inspection, and even after bias-field correction leave images unusable for computational analyses. Some sequences, e.g., MP2RAGE [2], were developed specifically to mitigate these B1-induced image variations. However, such sequences may have other shortcomings, e.g., prolonged scan time, not being automatically reconstructed at the scanner, or a different contrast than what a dedicated neuroradiologist at the local center prefers. It is therefore desirable to seek out stable solutions to this commonly encountered issue at 7T MRI with a classical setup. A simple and widely used method is to apply dielectric pads on each side of the head. But even with these, the spatial dependency of the B1+-field may induce signal dropout in mesial or lateral brain regions (figure 1), which may prevent optimal conditions for inspection or analyses. Such signal dropout and inhomogeneity may further vary with head size, positioning, image contrast and sequence type, and leaves clinicians and researchers with a highly varying image quality. We investigated the effects of combined use of large bilateral dielectric pads and 3D-based RF gain optimization on the stability of bias fields in 3D MPRAGE images. To compare across cohorts, we used the coefficient of variation of the computed bias field generated in post-processing of 3D MPRAGE scans.Methods
Informed consent was obtained according to local ethical guidelines, in relation to a larger scan protocol. 3D MPRAGE images (0.7 mm isotropic) were acquired on an actively shielded 7T MR system (Philips, Achieva, Best, The Netherlands), with a quadrature 32/2 Rx/Tx coil (Nova Medical, Wilmington, MA), and dielectric pads on both sides of the head.Ninety-five subjects were included, and divided into three groups: Group 1 (n=48) with smaller dielectric pads and 2D-based RF gain optimization, group 2 (n=29) with renewed, larger pads (19x19 cm, Multiwave Imaging, Marseille, France) and 2D-based RF gain optimization, and group 3 (n=18) with the renewed pads and 3D-based RF gain optimization. This was done by including a DREAM sequence to map the B1+ field (3.5mm x 3.5mm in-plane resolution, 36 slices, FOV: 240 mm x 180 mm x 240 mm, STEAM preparation flip angle: 35 deg.). In general, we observed ~40% variation in the B1+-maps, and a tendency of the RF gain to be overestimated (see figure 1). To counter-act this, we implemented an automated adjustment of the RF gain, based on the acquired DREAM map: After light smoothing and masking, the maximum B1+-intensity was identified and used for setting the RF gain. Bias fields were computed with bias-field correction of all MPRAGE images with step-size 3, FWHM 60 mm and light regularization (0.001) in SPM12. Subsequently, we computed a coefficient of variation (CV), defined as the ratio of the standard deviation to the mean of the bias-field intensity per axial slice inside a brainmask. We used ANOVA test to compare the per-subject average of CV (|CV|) between the three groups.
Results
As demonstrated in figure 2, there was a larger spread of CV across subjects in group 1, compared to group 2 and 3. There was a slight trend in increased spread of CV values in group 2 compared to group 3. Boxplots on figure 3 shows the distributions of |CV| in the three groups. ANOVA test revealed significant differences (p=1.7307e-21). This was largely driven by the variation in group 1, albeit group 2 and 3 was also found to be significantly different (p= 0.0387).Discussion
By comparing CV across groups in our study, we saw significant differences between different approaches for minimizing B1-induced bias fields. The combination of large pads and 3D-based RF gain optimization (group 3) yielded the smallest |CV|-variation, indicating increased stability in the bias field and thereby B1+. However, this is a quantitative and indirect measure of the quality-parameter “stability”, and thus may not be translatable to all subjects, nor in all contrast weightings. While we analyzed 3D MPRAGE, other sequences, e.g., 3D FLAIR, are even more vulnerable to B1+-induced variations, and would likely show larger effects. In our experience, the apparent quality improvement observed here are likewise seen for other sequences during radiological evaluation, and the implementation of the 3D-based RF gain optimization have completely mitigated cases of severe signal dropout in mesial brain regions across sequences.Conclusions
When performing structural neuroimaging at 7T with a classical coil setup and transmit mode (i.e., low RF transmit channel count), we recommend using a combination of large dielectric pads on each side of the head along with 3D-based RF gain optimizationAcknowledgements
The project is supported by the Independent Research Fund Denmark. The 7T scanner was donated by the John and Birthe Meyer Foundation and The Danish Agency for Science, Technology and Innovation (grant no. 0601-01370B).References
[1] Trattnig, S. et al. (2018). Key clinical benefits of neuroimaging at 7 T. Neuroimage, 168, 477-489.
[2] Marques, J. P. et al. (2010). MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage, 49(2), 1271-1281.
Figures
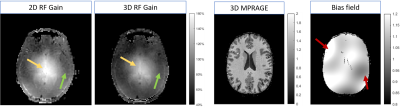
Figure 1:
Two images to the left: Examples of B1+ maps after 2D and 3D RF gain optimization from the same subject. The 2D gain optimization for this subject caused B1+ of 160% in the center of the brain. The 3D optimization caused B1+ of 140% (yellow arrows). The green arrows indicate lateral area typically presenting B1-induced inhomogeneity whose severity vary particularly with head size and placement of dielectric pads.
Two right images: Example of a bias field corrected 3D MPRAGE image and the computed bias field. The red arrows demonstrates 40% MPRAGE magnitude variations.
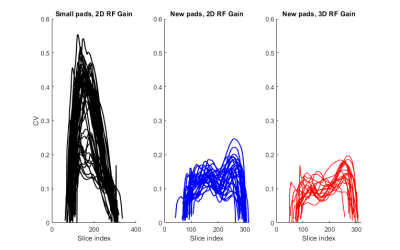
Figure 2:
Plots showing variability of CV across the slices in the individual brainmasks for group 1 (black, n=48), group 2 (blue, n=29) and group 3 (red, n=18). The slice-indexing is feet-head, i.e. high numbers corresponds to the top of the head.
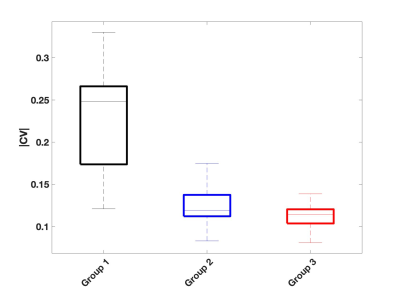
Figure 3:
Boxplot showing distributions of mean CV values in all three groups. The three groups were found to be significantly different.