1387
Ultra-wide-band radar for respiratory motion correction of T1 mapping in the liver1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany
Synopsis
In this work we introduce a motion correction approach based on a calibrated ultra-wide-band radar signal acquired simultaneously to the MR scan. Our method is contactless and entirely independent from scanner setups or applied imaging sequences which makes it a viable alternative to established motion correction approaches, like navigator sequences or breathholds. We could demonstrate in phantom and in-vivo scans that the proposed motion-correction approach strongly improves T1 quantification.
Introduction
T1 mapping of the liver can enable quantitative assessment of liver fibrosis and could also be important for classification of malignant lesions [1,2,3]. One of the main challenges is respiratory motion. Breathhold imaging has been proposed but this strongly limits scan times. In addition, if multiple breathholds are required to cover multiple slices, misalignments can occur due to varying breathhold positions[4].Respiratory motion correction overcomes these challenges but requires robust motion information. Here we propose to use an ultra-wide-band (UWB) radar surrogate for motion correction. In contrast to an MR-based navigator, it is not influenced by the MR data acquisition (e.g. inversion or saturation pulses required for T1 mapping). It can penetrate tissue and hence does not just provide a signal from the body surface such as a respiratory belt but measures organ motion inside the body[5].
The UWB signal is obtained simultaneously to the MR acquisition. In a first step a calibration scan is used to build a linear model between respiratory motion and UWB surrogate. This model is then applied to any following measurements, improving T1 quantification.
Methods
All imaging was carried out on a 3T scanner (MAGNETOM Verio, Siemens Healthineers, Erlangen, Germany) equipped with a large 4-channel Siemens Flex coil and an 8-channel Siemens Spine Matrix coil. The calibration was performed using a dynamic sagittal scan with 50 images over a duration of 20 seconds (TR/TE/FA 3.5ms/1.39ms/12°, GRAPPA (2x accel., 32 ref. lines), voxel size=1.2x1.2x8mm³). The data points for T1 mapping were acquired continuously over 16 seconds using a 2D golden angle trajectory with 3360 spokes and also in sagittal view. (TR/TE/FA 4.2ms/1.87ms/5°, voxel size=1.7x1.7x8mm³). Inversion pulses were applied after every 2.1s[6].Our UWB radar system is a MEODAT designed system consisting of a pair of MR-compatible double-ridged horn antennas for receiving and one for transmitting the radar signal, as well as an M-Sequence Baseband Module and a Cross Correlator, which provides an impulse response given the transmitted M-sequence and its corresponding received sensor response[7].
In a first step, a calibration scan is carried out while simultaneously collecting the radar impulse response functions. Rigid respiratory motion parameters are extracted from the dynamic images by registration of a manually defined region of interest placed around vessels in the liver with high contrast. The resulting translational shift gets fit to a spline function and resampled to match the temporal resolution of the radar (Δt=22.6ms). This is done for foot-head and anterior-posterior shifts, which have similar shapes. Via principal component analysis changes between the acquired radar spectra in time can be identified. These radar changes together with the translational shift in our dynamic images are linearly fitted to obtain a motion model for foot-head and anterior-posterior motion, respectively. Figure 1 gives an overview of the proposed method.
This model is used to estimate respiratory motion shifts during subsequent T1 measurements and apply the corrections retrospectively during image reconstruction and T1 mapping[8]. Motion correction and T1 mapping were evaluated in a moving T1 phantom and T1 measurements were performed on a healthy volunteer.
Results and Discussion
Figure 2 shows the first principle component obtained from the radar-based motion signal, the registered displacement of the liver along the foot-head direction and the fit of the calibrated motion model. High correlation between radar signal and registered displacement can be observed leading to an accurate motion model. Figure 3 demonstrates the proposed motion correction on a T1 phantom with 9 different tubes[9]. Motion correction improves visualization and quantification. In Figure 4 we can see an improvement of image quality in the in-vivo T1 maps, not only in the region used for calibration but in the entire liver and also the kidneysConclusion
We presented an UWB-radar-based motion correction approach to compensate for respiratory motion in the liver for T1 mapping. A calibrated radar response was used to retrospectively correct for respiratory motion. We were able to show that our approach resulted in enhanced image quality for T1 mapping both in phantoms and in-vivo scans. Currently our approach is limited to retrospective in-plane correction. Future work will focus on extending the correction to shifts in slice direction applied prospectively during data acquisition (slice tracking) to allow for scanning in arbitrary scan orientations.Acknowledgements
The results presented here have been developed in the framework of the 18HLT05 QUIERO Project. This project has received funding from the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.References
[1] Heye, T. et al. MR relaxometry of the liver: significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur. Radiol. 22, 1224–1232. (2012).
[2] Dekkers IA, Lamb HJ. Clinical application and technical considerations of T1 & T2(*) mapping in cardiac, liver, and renal imaging. Br J Radiol. 2018;91(1092):20170825.
[3] Kelekis, Nikolaos L., Richard C. Semelka, and John T. Woosley. "Malignant lesions of the liver with high signal intensity on T1‐weighted MR images." Journal of Magnetic Resonance Imaging 6.2 (1996): 291-294.
[4] Takamatsu, Shigeyuki, et al. "Reproducibility of diaphragm position assessed with a voluntary breath-holding device." Japanese journal of radiology 31.5 (2013): 357-363.
[5] Hilger, Ingrid, et al. "ultraMEDIS—ultra-wideband sensing in medicine." Ultra-Wideband Radio Technologies for Communications, Localization and Sensor Applications (2013): 296.
[6] Becker KM, et al., 2018. Simultaneous high-resolution cardiac T1 mapping and cine imaging using model-based iterative image reconstruction. Magn Reson Med, 2019; 81(2):1080-1091.
[7] Thiel, Florian, Olaf Kosch, and Frank Seifert. "Ultra-wideband sensors for improved magnetic resonance imaging, cardiovascular monitoring and tumour diagnostics." Sensors 10.12 (2010): 10778-10802.
[8] Becker KM, Blaszczyk E, Funk S, Nuesslein A, Schulz‐Menger J, Schaeffter T, Kolbitsch C. 2020 Fast myocardial T 1 mapping using cardiac motion correction. Magn. Reson. Med. 83, 438–451.
[9] Captur, Gabriella, et al. "A medical device-grade T1 and ECV phantom for
global T1 mapping quality assurance—the T 1 Mapping and ECV
Standardization in cardiovascular magnetic resonance (T1MES) program." Journal of cardiovascular magnetic resonance 18.1 (2016): 58.
Figures
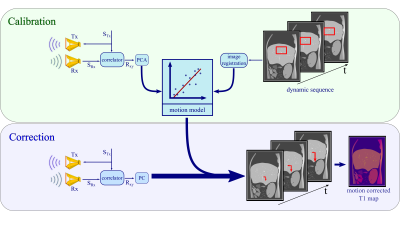
Figure 1: Calibration: The M-sequence STx gets transmitted by a sending antenna Tx and correlated with the received response SRx to create an impulse response Rxy. The principal components of Rxy get linearly fitted to the registered changes in a selected region of interest in the dynamic scan. Correction: Based on the motion model, radar signals obtained simultaneously to the T1 mapping sequence are used to predict respiratory motion shifts which can then be utilized during image reconstruction to correct for motion artefacts.
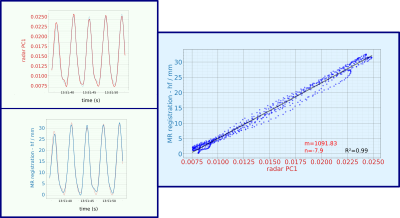
Figure 2: First principle component of the radar signal and registered foot-head shift show very good correlation leading to a high R2 of 0.99 using a linear motion model. Here only the results for foot-head (hf) translation are shown but similar results were obtained for anterior-posterior shifts albeit with smaller motion amplitudes.
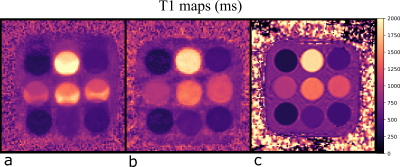
Figure 3: Scan of a phantom moving periodically up and down over a distance of 17mm (a) without and (b) with the proposed radar-based motion correction. Visualization of the individual tubes and T1 quantification were improved. (c) T1 map estimated with a MOLLI scan of the phantom at rest is shown for reference.

Figure 4: The motion corrected T1 maps show an increase in image quality. Respiratory blurring especially at the dome of the liver and around blood vessels could be reduced yielding similar image quality than the breathholdscan. Although the motion model was built for the liver, also the visualization of the kidneys is improved. It should be noted, that the breathhold data cannot be directly compared to motion corrected images, because they were acquired in two separate scans.