1385
Motion Correction of Abdominal Diffusion-Weighted MRI Using Internal Motion Vectors1Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3The Department of Radiology and Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mt. Sinai, New York, NY, United States
Synopsis
Respiratory motion is a significant problem in MRI and is further amplified in abdominal imaging. Traditional methods to correct for motion in abdominal DWI significantly increase overall scan time. In this work, motion vectors derived from non-rigid registration of low b-value diffusion volumes are used to correct for motion in higher b-values, where low signal to noise and contrast make intra and inter b-value registration challenging. Initial results suggest the proposed method can produce images similar in quality to respiratory gating, while maintaining reduced acquisition times.
Introduction
Respiratory motion is a significant problem in abdominal MRI (1). Many solutions currently exist to compensate for respiratory motion, including breath-holds (2), navigator gating (3–8), extra-dimensional MRI (9,10), and image registration (11–17). In the case of diffusion-weighted imaging, respiratory gating significantly extends the exam duration. As an alternative, image registration can be used; however, commonly applied 2D registration ignores through-plane motion which can have a significant effect on axial views. 3D image registration requires binning the individual DWI averages into discrete motion states prior to registration. However, low signal at higher b-values and varied contrast between b-values makes intra- and inter-b-value image registration challenging, respectively. In this study, we propose the use of pre-defined motion vectors derived from intra-registration of the low-b-value volumes (18,19) for motion correction of the higher b-values, resulting in significantly reduced scan time compared to prospectively gated acquisitions while maintaining similar image quality.Methods
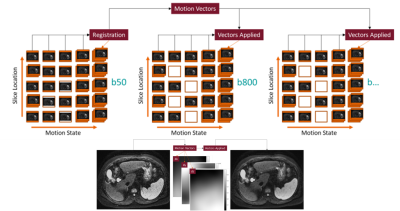
DWI iMoCoAll sampled averages of b50 are binned into 5 discrete respiratory motion states using a respiratory bellows for motion estimation and a hybrid binning strategy as shown in Figure 1; slices are organized with respect to slice position, and all binned averages are combined to create a contiguous b50 volume for each motion state. Each motion state is then registered to the end-expiratory volume using a rigid translation to remove bulk motion. To correct for non-rigid motion, each motion state is additionally registered to the end-expiratory volume using a diffeomorphic non-parametric algorithm (18,19), correcting for through- and in-plane motion for the final b50 volume. The rigid and non-rigid translations are stored with respect to each motion state and used to warp the b800 motion states. Resultant motion-corrected volumes are then aligned, and ADC maps are generated using a mono-exponential fit.
Acquisitions and Protocols
For all healthy and patient volunteers, a single dataset consisting of 16 3D-diagonal diffusion weighted averages for both b50 and b800 was acquired in an axial plane allowing for retrospective down-sampling of the number of averages to be used in the final reconstruction. Four test cases were reconstructed, denoted by the number of averages included as (a-b), where a and b are the number of averages for b50 and b800, respectively:
1.) Free breathing (1-5) (=1 b50 average, 5 b800 averages)
2.) End-expiratory gated (16-16)
3.) Free breathing (8-5)
4.) DWI iMoCo (8-5)
Motion vectors were calculated using the b50 averages and applied to the b800 images for correction. This method was applied in Test Case 4, where the 8 averages acquired for b50 are used to create motion vectors for application to the 5 b800 averages. In Test Case 2, end-expiratory gating is applied retrospectively to all 16 acquired averages, resulting in an average inclusion of (~7-6) motion-compensated averages of b50 and b800, respectively. The proposed method was first evaluated in a motion phantom (MP) experiment on a 3T system (MAGNETOM Prisma Fit, Siemens Healthcare, Erlangen, Germany), where a respiratory waveform was used to drive a programmable motion stage (Vital Biomedical) in the superior-inferior (SI) direction. Then, two healthy volunteers and 3 research subjects with liver disease and suspected portal hypertension (evaluated invasively by transjugular biopsy with hepatic venous pressure gradient (HVPG) measurements) were recruited for inclusion in this study. Healthy volunteer scans were performed on a 3T system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany), and patient scans were performed on a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). This study was approved by the local IRB, and written consent was obtained from each subject.
Results and Discussion
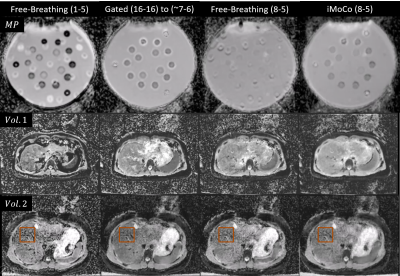
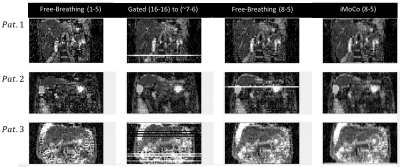
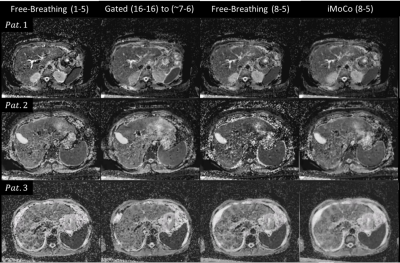
Reformatted coronal views from the MP experiment are shown in Figure 2. Free-breathing views show significant distortion and distension in the superior-inferior (SI) direction corresponding to the applied motion of the phantom; misaligned b50 and b800 images then produce similarly distorted and distended ADC maps. Increasing the averages included for b50 from one to 8 reduces the motion effect but does not eliminate the resultant blurring. iMoCo views show significantly reduced motion artifacts, while allowing for a reduced required acquisition time (TA = 1:47 min) compared to Gated (TA = 4:00 min) by reducing the number of averages included in the final reconstruction. Figure 3 shows original axial views for the MP and healthy volunteer experiments. Similar results can be observed, where free-breathing views show in-plane distortion that is improved with the use of iMoCo while maintaining a shorter acquisition time. Reformatted coronal views from the patient volunteer experiments are shown in Figure 4. Respiratory patterns in these subjects were highly variable, resulting in missing slices in the gated views. iMoCo maintains good image quality by using all acquired averages, ensuring no slice locations are missing. Figure 5. shows original axial views for the patient volunteers, further illustrating the ability of iMoCo to reduce motion artifacts.Conclusion
iMoCo has reduced the effects of respiratory motion, while maintaining similar image quality to the end-expiratory-gated gold standard. These results imply that the use of iMoCo could increase the clinical applicability of free breathing quantitative diffusion imaging of the abdomen, by improving the workflow efficiency of the acquisition and the reproducibility of the resultant ADC maps.Acknowledgements
No acknowledgement found.References
1. Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging 2015;42:887–901. doi: 10.1002/jmri.24850.
2. Chavhan GB, Babyn PS, Vasanawala SS. Abdominal MR imaging in children: Motion compensation, sequence optimization, and protocol organization. Radiographics 2013. doi: 10.1148/rg.333125027.
3. Chuang ML, Chen MH, Khasgiwala VC, McConnell M V., Edelman RR, Manning WJ. Adaptive correction of imaging plane position in segmented k-space cine cardiac MRI. J. Magn. Reson. Imaging 1997;7:811–814. doi: 10.1002/jmri.1880070507.
4. Weingärtner S, Roujol S, Akçakaya M, Basha TA, Nezafat R. Free-breathing multislice native myocardial T1 mapping using the slice-interleaved T1 (STONE) sequence. Magn. Reson. Med. 2015;74:115–124. doi: 10.1002/mrm.25387.
5. Manke D, Nehrke K, Börnert P, Bo P, Börnert P, Bo P. Novel prospective respiratory motion correction approach for free-breathing coronary MR angiography using a patient-adapted affine motion model. Magn. Reson. Med. 2003;50:122–131. doi: 10.1002/mrm.10483.
6. Firmin D, Keegan J. Navigator echoes in cardiac magnetic resonance. J. Cardiovasc. Magn. Reson. 2001;3:183–193. doi: 10.1081/JCMR-100107467.
7. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173:255–263. doi: 10.1148/radiology.173.1.2781017.
8. Wang Y, Riederer SJ, Ehman RL. Respiratory Motion of the Heart: Kinematics and the Implications for the Spatial Resolution in Coronary Imaging. Magn. Reson. Med. 1995;33:713–719. doi: 10.1002/mrm.1910330517.
9. Correia T, Ginami G, Cruz G, Neji R, Rashid I, Botnar RM, Prieto C. Optimized respiratory-resolved motion-compensated 3D Cartesian coronary MR angiography. Magn. Reson. Med. 2018;80:2618–2629. doi: 10.1002/mrm.27208.
10. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016;75:775–788. doi: 10.1002/mrm.25665.
11. Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn. Reson. Med. 2012;67:1644–1655. doi: 10.1002/mrm.23153.
12. Usman M, Atkinson D, Odille F, Kolbitsch C, Vaillant G, Schaeffter T, Batchelor PG, Prieto C. Motion corrected compressed sensing for free-breathing dynamic cardiac MRI. Magn. Reson. Med. 2013;70:504–516. doi: 10.1002/mrm.24463.
13. Kellman P, Chefd’hotel C, Lorenz CH, Mancini C, Arai AE, McVeigh ER. Fully automatic, retrospective enhancement of real-time acquired cardiac cine MR images using image-based navigators and respiratory motion-corrected averaging. Magn. Reson. Med. 2008;59:771–778. doi: 10.1002/mrm.21509.
14. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn. Reson. Med. 2016. doi: 10.1002/mrm.25708.
15. Buerger C, Clough RE, King AP, Schaeffter T, Prieto C. Nonrigid motion modeling of the liver from 3-D undersampled self-gated golden-radial phase encoded MRI. IEEE Trans. Med. Imaging 2012. doi: 10.1109/TMI.2011.2181997.
16. Ingle RR, Wu HH, Addy NO, Cheng JY, Yang PC, Hu BS, Nishimura DG. Nonrigid autofocus motion correction for coronary MR angiography with a 3D cones trajectory. Magn. Reson. Med. 2014;72:347–361. doi: 10.1002/mrm.24924.
17. David, Christian; Vahle, Thomas; Grimm, Robert; Bachert, Peter; Kachelries M. Motion Compensation for Free-Breathing Diffusion-Weighted Imaging (MoCo DWI). ISMRM 2020 2020;3397.
18. Thirion JP. Image matching as a diffusion process: An analogy with Maxwell’s demons. Med. Image Anal. 1998. doi: 10.1016/S1361-8415(98)80022-4.
19. Vercauteren T, Pennec X, Perchant A, Ayache N. Diffeomorphic demons: efficient non-parametric image registration. Neuroimage 2009. doi: 10.1016/j.neuroimage.2008.10.040.
Figures