1381
Respiratory resolved and corrected 3D $$$\Delta\text{B0}$$$ mapping and fat-water imaging at 7 Tesla1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berin, Germany, 2Charité Medical Faculty University Medicine, Berlin, Germany, 3DZHK partner site Berlin, Working Group on Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center (ECRC), Berlin, Germany, 4Department of Cardiology and Nephrology, HELIOS Klinikum Berlin Buch, Berlin, Germany, 5Department of Medical Engineering, Technische Universität Berlin, Berlin, Germany, 6University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States
Synopsis
This work demonstrates 3D respiratory motion-corrected and cardiac resolved fat-water separation in the human body at 7T. Accurate fat fraction quantification using bipolar readout gradients was validated in a phantom. The impact of motion compensation on the underlying motion resolved $$$\Delta\text{B0}$$$ maps is investigated and estimated. In total the 3D fat-water separation and fat fraction quantification is successfully demonstrated in 10 healthy volunteers at 7T with a large BMI range from $$$19$$$ to $$$34 \text{kg/m}²$$$.
Introduction
Fat-water imaging of the entire heart benefits from ultra-high fields (UHF) due to higher SNR and better spectral separation of the chemical components. However, at UHF the inhomogeneous spatial distribution of the transmit (Tx) magnetic field ($$$\text{B}_1^+$$$) can lead to contrast variations and to signal cancellation1. Furthermore, the respiratory motion, cardiac motion, and blood flow can lead to artifacts and increased acquisition times for 3D cardiac magnetic resonance (CMR) images. At lower fields, respiration-induced artifacts are commonly reduced by using 1D/2D respiratory navigators. At UHF, however, such navigators often fail due to signal cancellations2,3. Alternatively, breath holds scan can be performed for 2D scans, but this is likely to fail for 3D acquisition due to scan time limitations.In this work, we investigate the feasibility of a free-breathing 3D whole heart fat-water imaging approach introduced at 1.5T4 and additionally generate 3D respiration resolved ΔB0 maps for use at 7T. A radial phase encoding (RPE)5,6 acquisition allowed for respiratory self-navigation, non-rigid motion correction and retrospective cardiac binning for accurate fat quantification at (1.4mm)³ isotropic resolution.
Methods
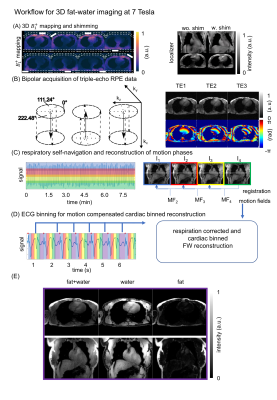
All scans were performed on a 7T with 8-channel pTx system (Magnetom 7T, Siemens, Germany) and a 32-element torso coil array (MRI.TOOLS, Berlin, Germany) driven in 8Tx/32Rx mode. RF power of each Tx channel was restricted to meet SAR limits. In-vivo measurements were performed according to an IRB approved protocol. A phantom filled with water, $$$2\%~$$$agarose, $$$0.2\%~$$$NaCl, $$$0.1\%~$$$CuSO4 ($$$σ=0.4\,\text{S/m}, ε_r=80$$$) containing three tubes filled with a $$$25\%/50\%/100\%$$$ olive-oil emulsion in agarose gel was used to validate the acquisition and fat-water reconstruction at 7T. Ten healthy volunteers ($$$7\text{m}$$$/$$$3\text{f}$$$, $$$21\text{-}35\text{y}$$$, mean:$$$30\text{y}$$$) with a wide BMI range ($$$19.9\text{-}34.0\,\text{kg/m}²,~\text{mean:}23.9\,\text{kg/m}$$$) were scanned in the supine position. Fig.1 shows the applied workflow to achieve respiratory motion-corrected and cardiac resolved 3D fat-water images at 7T.3D non-respiratory resolved $$$\text{B}_1^+$$$ maps obtained under shallow breathing were acquired in all subjects (see Fig.1(A)) with nominal flip angle ($$$\text{FA}$$$) of $$$20°$$$ (echo/repetition time ($$$\text{TE/TR}$$$)$$$~$$$of$$$~2.0/40.0\,\text{ms}$$$, field-of-view $$$\text{(FOV)}~$$$of$$$~250\times\,312\times\,312\,\text{mm}^3$$$,$$$~\text{resolution}=(4\,\text{mm})^3$$$) with an acquisition time ($$$\text{TA}$$$) of$$$~3.41\,\text{ms}$$$ to obtain a homogeneous subject-specific phase-only shim for the human heart.7
Subsequently, a triple-echo 3D spoiled gradient echo (GRE) acquisition with RPE trajectory and bipolar readout was acquired during free-breathing covering a$$$~\text{FOV}=250\times\,312\text{-}350\times\,312\text{-}350\,\text{mm}^3$$$ with $$$(1.4\,\text{mm})^3$$$ isotropic resolution ($$$\text{TE1/TE2/TE3/TR}=1.51/2.79/4.07/6.10\,\text{ms}$$$, actual $$$\text{FA}\approx5\,°$$$7) in $$$\text{TA}=9.17\,\text{min}$$$ (see Fig.1(B)).
First, three datasets with different TEs were reconstructed without any motion compensation to determine the bipolar readout correction factors. Subsequently, data from the first echo was split into four respiratory states using self-navigation and reconstructed using iterative SENSE8. 3D non-rigid motion vector fields (MF) describing the pixels displacements relative to end-expiration are derived from these respiration-resolved datasets9 (see Fig.1(C)). These MF were used for motion-corrected image reconstruction10 for all TEs. Furthermore, respiratory-motion corrected and respiration-resolved data is used to obtain $$$\Delta\text{B0}$$$ maps as described in ref. 11. Respiration-resolved data reconstruction included a total variation constraint along the respiratory dimension. Then, the bipolar and respiratory motion-corrected GRE data was split into five different cardiac phases using the recorded ECG signal (see Fig.1(D)). The acquisition window of each phase depends on the heart rate and corresponds to $$$\approx 200\,\text{ms}$$$ per cardiac cycle and the acceleration factor per bin is $$$\text{R}=3.2$$$. Therefore, a total-variation-based regularization12 along the cardiac phases and spatial position in combination with a 6-peak spectral model12 of fat was used to reconstruct directly respiratory motion-corrected and cardiac resolved fat (F) and water (W) magnitude information (see Fig.1(E)). Fat fraction ($$$\text{FF}$$$) images were calculated by $$\text{FF}=\frac{\text{F}}{\text{W}+\text{F}}\,.$$
Results/Discussion
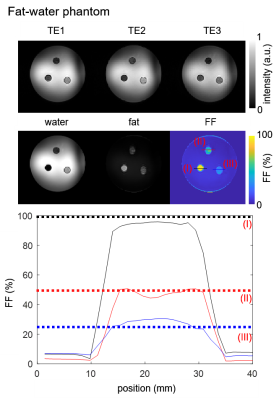
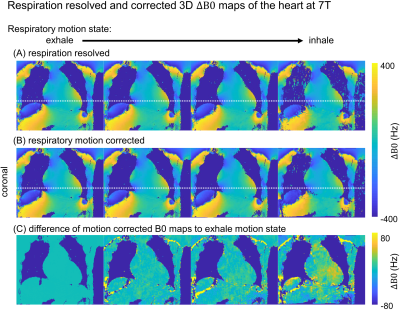
Fig.2 shows phantom images at the three different echo times and corresponding water, fat, and FF images. The line plot indicates a reasonable match between mixed oil/water-agarose fraction and MR quantification in the tubes with mean (standard deviation) values over the entire volume of $$$28(7)\%/45(7)\%/92(5)\%$$$ for the $$$25\%/50\%/100\%$$$ fat compartments.Fig.3 illustrates reconstructed respiration-resolved (A) and respiration-corrected (B) $$$\Delta\text{B0}$$$ maps with $$$\Delta\text{B0}=\pm90\,\text{Hz}$$$ averaged over the whole heart in end-expiration. Similar 2D $$$\Delta\text{B0}$$$ maps have been shown by ref.13 for a default $$$\Delta\text{B0}$$$ shim setting. Absolute differences between end-expiration and motion-corrected respiratory phases (C) increase from end-expiration to end-inspiration with a maximum difference of $$$\pm\,24\,\text{Hz}$$$ for the whole heart. Such changes are regarded as small compared to the overall $$$\Delta\text{B0}$$$ range of $$$[-300\,\text{-}\,300\,\text{Hz}]$$$ which justifies the use of respiratory motion-corrected information for fat-water separation.
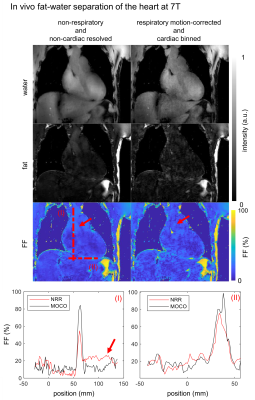
Fig.4 shows non-respiratory and non-cardiac resolved and respiratory motion-corrected, cardiac resolved (diastole) fat-water, and $$$\text{FF}$$$ images of the heart. Dotted lines indicate the spatial position of line plot data and the red arrows indicate flow artifacts in both, $$$\text{FF}$$$ maps and line plot. Quantitatively, local $$$\text{FF}$$$ is increased up to 24% and the $$$\text{FF}$$$ in the blood pool is reduced by the factor of two due to the reduced blood flow in the reconstructed diastolic phase.
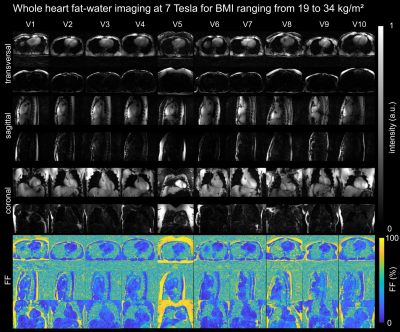
Fig.5 illustrates the results of all 10 volunteers in 3 orientations with corresponding $$$\text{FF}$$$ with successful fat/water separation.
Conclusion
In this work, we demonstrate respiratory motion-corrected 3D cardiac fat-water images and $$$\Delta\text{B0}$$$ maps at 7T without $$$\text{B}_1^+$$$-related signal voids within the heart and overall good image quality. This work extends the field of CMR applications at 7T and it may help to push future 7T body imaging, also for other organs.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1 and GRK2260, BIOQIC.References
1. Padormo, Beqiri, Hajnal, Malik, NMR in Biomedicine 29:1145-1161 (2016)
2. Ladd, Bachert, Meyerspeer, Moser, Nagel et al., Prog Nucl Magn Reson Spectrosc 109:1–50 (2018).
3. Schmitter, Schnell, Uğurbil, Markl, Van de Moortele, JMRI 44:486–99 (2016)
4. Kolbitsch, Mayer, Cipriani, Blaszczyk, Schulz-Menger et al., Proc ISMRM 27, Abstract 0402 (2019)
5. Prieto, Uribe, Razavi, Atkinson, Schaeffter, MRM 68:514-526 (2010)
6. Buerger, Clough, King, Schaeffter, Prieto, Trans Med Imaging 31: 805-815 (2012)
7. Dietrich, Aigner,
Kolbitsch, Ludwig, Mayer et al., MRM DOI:10.1002/mrm.28602 (2020)
8. Pruessmann, Weiger, Peter, Boesiger, MRM 46:638–51, (2001)
9. Jackson, Meyer, Nishimura, Macovski, IEEE Transactions on Medical Imaging 10: 473–478 (1991)
10. Kolbitsch, Bastkowski, Schäffter, Prieto, Weiss, et al., MRM 83:635–44 (2020)
11. Berglund, Johansson, Ahlström, Kullberg, MRM 63:1659-1668, (2010)
12. Benkert, Feng, Sodickson, Chandarana, Block KT, MRM 78:565–76, (2017)
13. Hock, Terekhov, Stefanescu, Lohr, Herz, Reiter et al., MRM 85:182–96, (2021)
Figures