1375
Dual-echo volumetric navigators for field mapping and shim correction in MR neuroimaging1Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
We demonstrate the feasibility and validity of field mapping using a dual-echo vNav based on a fly-back EPI readout. A human subject was scanned using the proposed method and a standard FLASH acquisition for comparison. The proposed method does not require additional reconstruction or acquisition complexity and is easily computed on typical MR scanners to support real-time motion and shim correction.
Introduction
The use of navigators in MRI is one method of motion tracking that does not require the use of additional hardware. In particular, volumetric navigators (vNavs) have been shown to enable accurate prospective and retrospective motion correction with negligible change in contrast and intensity of the parent sequence in neuroimaging.1, 2, 3, 4 To account for the significant changes in off-resonance that occur with motion,5 multiple navigator echoes can be used to generate field maps.1 Previous dual-echo approaches, while effective, require too much time to fit reasonably in standard MPRAGE protocols.1 We propose to accelerate the dual-echo vNav strategy by acquiring a highly undersampled second “fly-back” echo within each EPI partition train. We demonstrate this method’s validity by comparing it to field maps from a standard multi-echo FLASH scan.Methods
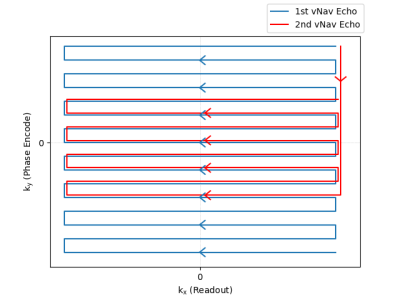
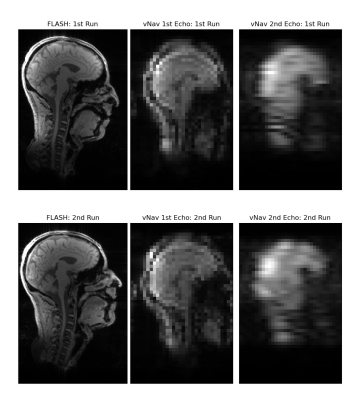
The first navigator echo was acquired as in existing vNav sequences, using 3D-encoded EPI with a matrix size of 32x32x32.2 A second vNav echo was implemented by acquiring an additional 8 lines after a flyback past the ky=0-axis, as illustrated in Figure 1 for one partition. This ensured that the directionality of the second echo matched the first for similar eddy current effects and matched distortion. The TEs of the first and second echoes were 6.685 ms and 12.545 ms, respectively, giving a difference of 5.86 ms, which enables off-resonance measurement up to +/- 85 Hz without wrapping. vNavs are normally acquired with an additional partition prepended to collect phase-correction and frequency drift information.6 In our novel navigator, this first partition acquired 32 reference lines for the first echo and 8 additional reference lines for the second echo by using the same trajectory as in Figure 1, except with phase encode blipping turned off. EPI ghost correction was performed by estimating even/odd line phase modulation using Wiener filters based on the temporal noise variance in the vNav data. Field maps were generated by dividing the phase difference between the echoes by the TE difference.Our dual-echo vNavs were embedded in a custom multi-echo MPRAGE (MEMPRAGE) sequence, and evaluated in a single human subject, who provided prior informed consent, in a 3 T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using the vendor’s 64-channel head and neck coil. Our protocol included both the proposed dual-echo vNav MEMPRAGE sequence, and a multi-echo FLASH scan for comparison. The FLASH scan had a TR of 25 ms and TEs of 4.92, 9.84, 14.76, and 19.68 ms, and the first and third echoes were used to generate a field map comparison. The FLASH echo spacing was chosen to keep fat and water at the same resonance offset for a 3 T field strength. The vNavs, by contrast, used a water-selective excitation and so should have less effect from fat phase differences between echoes. Two runs were performed: the first with the subject's head held in a fixed neutral position, and the second with the subject's head held in a fixed upward-extended position. Sagittal views of these two runs are shown in Figure 2 for the higher-resolution FLASH scan as well as for both the first and second vNav echoes.
Results
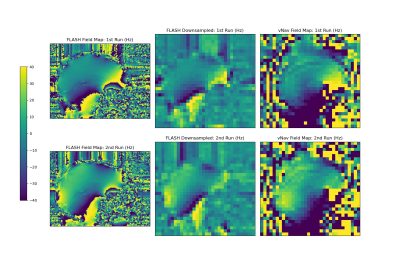
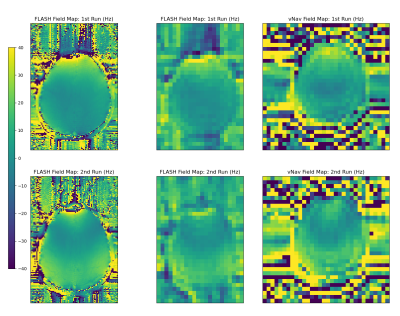
Magnitude images of the second vNav echo in Figure 2 show expected blurring in the anterior-posterior phase encode direction due to the lower traversal of k-space using only 8 phase encode lines. However, we are interested only in whether this lower resolution is sufficient for the global off-resonance and first order shim correction that standard MR scanners typically allow dynamically during scanning.Figures 3 (sagittal) and 4 (axial) show the field maps computed from the proposed dual-echo vNav sequence as well as from the FLASH scan, for each of the first and second runs. The FLASH field maps were downsampled by a factor of 4 to match the resolution of the vNavs for easier comparison. In the FLASH field map, the second run clearly shows a change between runs with the posterior aspect of the brain going from around 0 Hz in the first run to 30-40 Hz in the second run. The vNav field maps match this behavior closely both in a quantitative and qualitative sense, with a similar increase of 30-40 Hz between runs in that region and a similar change in overall shape of the field map between runs.
Discussion and Conclusion
We have demonstrated the feasibility and validity of field mapping using a dual-echo vNav for off-resonance and first order shim correction by the acquisition of 8 additional phase encode lines in a 32x32x32 3D EPI sequence. Unlike parallel-accelerated strategies for shortening dual-echo acquisitions, the proposed method does not require additional reconstruction or acquisition complexity and is easily computed on typical MR scanners for real-time motion and shim correction. In addition, the proposed method enables global off-resonance measurement based on a desired region of interest, unlike standard non-selective methods currently in use.7 Future work will explore the additional technical challenges of real-time parallel reconstruction. Our goal will be to further accelerate the first and second vNav echos, reducing both the delay between TE1 and TE2 in the navigator, and our overall navigator duration, making it comparable to current single-echo vNavs.Acknowledgements
The project described was supported by RSNA Research & Education Foundation through grant number RR2014 and by NIH award R34DA050297. The content is solely the responsibility of the authors and does not necessarily represent the official views of the RSNA R&E Foundation.References
1. Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, van der Kouwe AJW. Real-time motion and B0 corrected single voxel spectroscopy using volumetric navigators. Magn Reson Med. 2011;66(2):314–323.
2. Tisdall MD, Hess AT, Reuter M, et al. Volumetric Navigators for Prospective Motion Correction and Selective Reacquisition in Neuroanatomical MRI. Magn Reson Med. 2012;68:389-399.
3. Jiaen Liu, Peter van Gelderen, Jacco A. de Zwart, Jeff H. Duyn. Reducing motion sensitivity in 3D high-resolution T2*-weighted MRI by navigator-based motion and nonlinear magnetic field correction. NeuroImage 2020;206:116332.
4. Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T: 3D FatNavs for High-Resolution Retrospective Motion Correction. Magnetic Resonance in Medicine. 2016 Mar;75(3):1030–1039.
5. Jiaen Liu, Jacco A. de Zwart, Peter van Gelderen, et al. Effect of head motion on MRI B0 field distribution. Magn Reson Med. 2018;80(6):2538-2548.
6. Tisdall MD, van der Kouwe AJW. High-accuracy off-resonance estimation from EPI, with application to volumetric navigators (vNavs) enabling real-time motion and frequency correction. Proc Intl Soc Mag Reson Med. 2014. p. 1621.
7. Hoffman M, Frost R, Salat DH, et al. Real-time brain masking algorithm improves motion tracking accuracy in scans with volumetric navigators (vNavs). Proc Intl Soc Mag Reson Med. 2020. p. 3367.
Figures