1374
Prospective Motion Corrected Time-of-flight MR Angiography at 3T1Diagnostic Radiology, University of Maryland-Baltimore, Baltimore, MD, United States
Synopsis
MRA at 3T is sensitive to patient motion which sometimes occur due to severe illness. This work investigated the effect of optic prospective motion correction (PMC) on the MRA at 3T. MRA with optic PMC was tested in phantom and on a healthy volunteer and compared with MRA without PMC. The use of PMC successfully reversed the slab misalignment, artifactual stenosis, and discontinuous major arteries caused by the intentional motion by the volunteer, and enhanced the distal vessels. This study demonstrated the potential of optic PMC in improving the quality of MRA on patients with difficulty holding still.
Purpose
Time-of-Flight (TOF) of MR Angiography (MRA) is a standard non-invasive diagnostic tool to assess the neuro-vasculature. Due to typical acquisition times of several minutes and sub-mm spatial resolution, subjects are required to hold still for several minutes during acquisitions, which may not be possible for ill patients. Prior studies demonstrated that prospective motion correction (PMC) using in-bore cameras to track head motion1 can effectively reduce motion-induced MRA degradation at 1.5T2. Similarly, camera-based PMC was able to improve the quality of ultra-high resolution (~200μm) MRA scans at 7T3. However, to our knowledge, there are no studies of PMC for MRA at 3T, which has the advantages of wide availability, moderate examination times compared with ultra-high resolution at 7T, and much improved background suppression compared with 1.5T. The purpose of this study is to investigate the effect of camera-based PMC on image quality and diagnostic value of TOF MRA at 3T.Methods
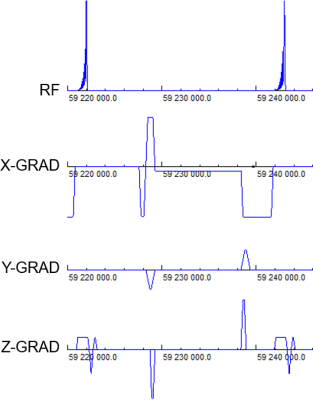
The experiments were performed on a 3T Prisma MR scanner (Siemens, Erlangen, Germany). PMC was performed using the KinetiCor platform (San Diego, CA), which uses four in-bore cameras and custom markers to track head motion (accuracy 0.1mm/degree; 60 frames/second). Phantom studies used a single marker, and the in-vivo experiment used two markers attached to the bridge of the nose.A water phantom was scanned using a TOF MRA sequence with custom motion updates (Figure 1). The acquisition parameters are as follows: FOV 300×300mm2, resolution 1.2×1.2×3.1mm3, acquisition matrix 256×256×32 with linear k-space filling, 1 slab, 32 slices of 2mm, TR/TE 21/10ms, flip angle 20°, acquisition time ~3 minutes. Following a reference scan without motion, the phantom was rescanned twice, once with and once without PMC, while being rotated about the x-axis by approximately 5° at 45 seconds into the scan.
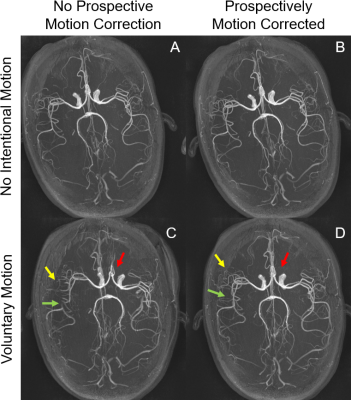
A healthy volunteer was recruited into a protocol approved by the local IRB, and gave written and verbal consent. Imaging parameters were: FOV 200×200×mm2, acquisition matrix 384×348×32, resolution 0.5×0.5×0.5mm2, 5 slabs with 40 slices each, slice thickness 0.5mm, TR/TE 21/3.42ms, flip angle 18°, acquisition time ~7 minutes. Prior to the scan, the subject was taught to perform a motion of ~7° rotation about the z-axis (moving head right-to-left). The MRA scan was performed four times: a) PMC off, no intentional motion; b) PMC on, no intentional motion; c) PMC on, trained motion; d) PMC off, trained motion. For the 2 motion scans, motion was initiated verbally by the scanner operator ~3½ minutes into each scan. An experienced neuroradiologist rated the quality of each scan on a scale from 1 to 5, with 1 indicating severe motion artifacts, image non diagnostic, and 5 indicating perfect delineation of arteries. The neuroradiologist was blinded to the four scan conditions.
Results
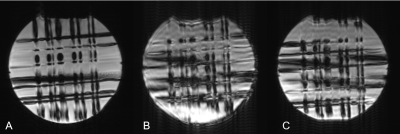
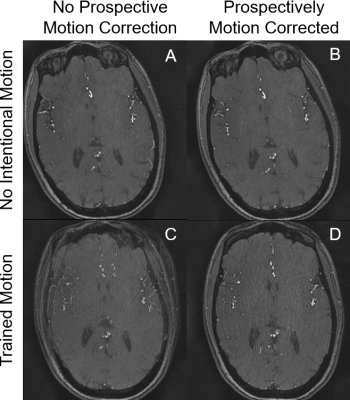
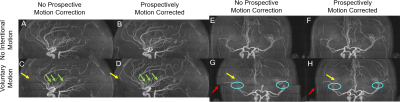
The phantom study with motion and no correction is shown in Figure 2A. PMC alleviated motion artifacts, but some residual blurring and ringing can still be observed (Figure 2B). In the human studies without intentional motion, MRA image quality was high regardless of the status of PMC (Figure 4A,4B,4E,4F,5A,5B,). Without PMC, the trained motion (single rotation) caused ghosting artifacts in the source images of the center slab (Figure 2 C). Distal vessels were also obscured on maximum intensity projections (MIP), as demonstrated in Figure 4C, 4G, 5C (yellow arrow). Additionally, motion caused slabs and arteries to be misaligned in the sagittal projection (Figure 4 G, red arrow, cyan circle). In comparison, PMC markedly improved the depiction of distal vessels (Figure 4H, 5D, yellow arrow), and reversed the misalignment between large arteries (Figure 4H, 5D, red arrow, cyan circle) and slabs (Figure 4, red arrow). The neuroradiologist rated the two scans without intentional motion 4 (very high quality). The MRA source images and MIPs with trained motion but no PMC were rated 2 (fair quality, limited diagnostic information). For instance, artifactual stenoses were observed on the MRA images with voluntary motion and no PMC (Figure 4C, green arrow). Conversely, PMC improved the rating of scans with intentional motion to 3 (good quality), and also reduced the occurrence of erroneous findings, such as pseudostenosis (Figure 4D, green arrows).Discussion
In this study, camera-based PMC effectively reduced motion artifacts and improved the diagnostic value of MRA images in the presence of motion. Since MRA is often performed in physically ill patients, sudden movements during the examinations are common in the clinic. We attempted to mimic a clinically relevant sudden movement in the middle of an MRA scan, and demonstrated that improves the quality of the resulting images. Some motion artifacts that may lead to false findings, such as pseudostenosis or arterial displacement, were eliminated.Compared with the motion corrected MRA study at 1.5T,2 our angiograms at 3T showed substantially better depiction of distal vessels due to stronger background suppression and higher image resolution. While image quality was even better at 7T,3 the 7T study focused on achieving the highest possible resolution in healthy volunteers with only minimal motion.
Conclusion
This study demonstrates the potential of camera based PMC in improving the quality of MRA scans in in subjects who move substantially during the scan.Acknowledgements
This work was supported by NIH grant 1R01 DA021146 (BRP).
We also would like to acknowledge Dr. Pan Su from Siemens Healthineers for providing technical assistance and expertise.
References
1. Maclaren J, Armstrong BSR, Barrows RT, et al. Measurement and Correction of Microscopic Head Motion during Magnetic Resonance Imaging of the Brain. PLOS ONE. 2012;7(11):e48088. doi:10.1371/journal.pone.0048088
2. Kopeinigg D, Aksoy M, Forman C, et al. Prospective optical motion correction for 3D time-of-flight angiography. Magnetic Resonance in Medicine. 2013;69(6):1623-1633. doi:https://doi.org/10.1002/mrm.24423
3. Mattern H, Sciarra A, Godenschweger F, et al. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magnetic Resonance in Medicine. 2018;80(1):248-258. doi:https://doi.org/10.1002/mrm.27033
Figures