1372
3D rigid motion correction for navigated interleaved simultaneous multi-slice DWI1Institute for Signal Processing, University of Lübeck, Lübeck, Germany, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Harvard‐MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 5Philips Research, Hamburg, Germany, 6Department of Radiology, C.J. Gorter Center for High-Field MRI, Leiden University Medical Center, Leiden, Netherlands
Synopsis
This work aims at improving the robustness of diffusion-weighted imaging (DWI) against 3D rigid head motion based on simultaneous multi-slice (SMS), interleaved echo-planar imaging (EPI) and image navigators. The proposed method utilizes low-resolution navigators to estimate diffusion phases as well as shot-wise 3D rigid motion through SMS-to-volume registration; providing high temporal resolution motion correction capability. The shot parameters are included into a full-volume reconstruction of the image data per diffusion direction. The method achieves submillimeter registration errors and improved image quality. In conclusion, the presented method enables retrospective 3D rigid motion correction for interleaved SMS DWI.
Introduction
Multi-shot DWI techniques are entering clinical practice overcoming current technical limitations in resolution and SNR of the prevalent single-shot EPI acquisitions1. The motion sensitive and rather lengthy multi-shot diffusion-weighted scans suffer from shot phase variations and gross motion1. In-plane gross motion can be addressed by dedicated model-based image reconstructions2, but through-plane motion remains challenging. At the same time, simultaneous multi-slice (SMS) acquisitions with controlled aliasing schemes offer scan time reductions at moderate g-factor penalty for interleaved EPI3,4 and provide data support for motion estimation in the through-plane direction per EPI-shot. Recently, SMS-to-volume registration approaches have been proposed for fMRI5 and DTI modeling6 to obtain and correct for shot-wise 3D rigid motion parameters. In this work, we introduce such a method for DWI image reconstruction and propose an algorithm, termed motion-aware SMS-accelerated and interleaved image creation (MoSaIC), with navigated 3D rigid motion correction in the brain.Methods
Image Reconstruction ModelAn SMS extension4 of a navigated Stejskal-Tanner sequence7 is used to acquire interleaved high-resolution image data at the first spin echo and moderately undersampled low-resolution navigator data at the second. The SENSE-based8 data discrepancy functional for interleaved SMS DWI is stated as
$$\underset{\boldsymbol{\rho}}{\mathrm{minimize}}\quad\lVert{M}\,{F}\,{\Theta}\,{C}\,{\Phi}\,{H}\,{\Omega}\;{\boldsymbol{\rho}}-\boldsymbol{b}\rVert_2^2 + {\alpha}\,\lVert{W}\boldsymbol{\rho}\rVert_2^2,$$ where $$$\boldsymbol{\rho}\in\mathbb{C}^{N_p}$$$ is the image volume with $$$N_{p}=N_{x}N_{y}N_{z}$$$ pixels and $$$\boldsymbol{b}\in\mathbb{C}^{N_b}$$$ is the acquired k-space data. The forward model contains the macroscopic motion operator $$$\Omega$$$ ($$$N_{shots}N_p{\times}N_p$$$) applying the shot transformation. The slice sampling operator $$$H$$$ ($$$N_{shots}N_{MB}N_{y}N_{x}{\times}N_{shots}N_{p}$$$) selects the $$$N_{MB}$$$ SMS slices per shot. The SMS slices are weighted by the diffusion phases using $$$\Phi$$$ ($$$N_{shots}N_{MB}N_{y}N_{x}{\times}N_{shots}N_{MB}N_{y}N_{x}$$$) and the $$$N_{c}$$$ coil sensitivities using $$$C$$$ ($$$N_{c}N_{shots}N_{MB}N_{y}N_{x}{\times}N_{shots}N_{MB}N_{y}N_{x}$$$). The CAIPI operator $$$\Theta$$$ ($$$N_{c}N_{shots}N_{y}N_{x}{\times}N_{c}N_{shots}N_{MB}N_{y}N_{x}$$$) applies CAIPI shifts and adds the SMS slices. For EPI (with gridded ramp samples), $$$F$$$ applies the Fourier transform along the phase-encoding direction and $$$M$$$ the sampling mask ($$$N_{b}{\times}N_{c}N_{shots}N_{y}N_{x}$$$). A weighted Tikhonov regularization is added with parameter $$$\alpha$$$ and a weight matrix $$$W=\mathrm{diag}(w_{p}^{-1})$$$ with a reference image $$$\boldsymbol{w}\in\mathbb{R}^{N_p}$$$. The shot parameters for $$$\Phi$$$ and $$$\Omega$$$ are estimated from low-resolution image navigator data, sampled for each SMS interleaf7, so that the full-volume reconstruction is convex.
Navigated Algorithm
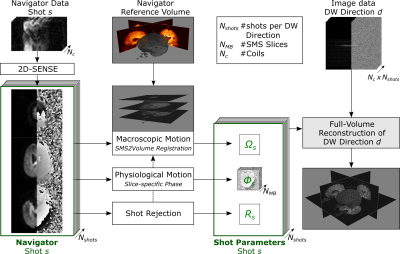
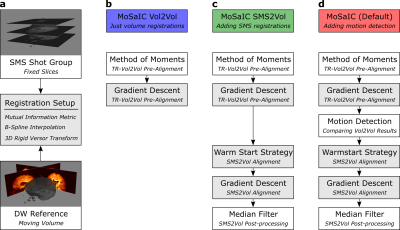
The algorithm, visualized in Fig. 1, comprises three main steps, namely: navigator reconstruction, shot parameter estimation from the navigators, and full-volume reconstruction. First, the navigator data, which is undersampled in phase-encoding and slice direction, is unfolded by 2D-SENSE9. Second, the simultaneous navigator slices are registered to a reference volume by a SMS-to-volume (SMS2Vol) registration scheme, which is implemented in SimpleITK10 and shown schematically in Fig. 2, yielding the 3D rigid shot motion parameters. The low-resolution navigator phases are extracted for shot- and slice-wise physiological motion correction. Shots whose l2-norm energy of the navigator signal drops below 5 times the median absolute deviation over all diffusion-weighted shots are rejected.
The full-volume reconstruction is solved by conjugate gradients with intensity correction8. The rigid motion operator is constructed as proposed by Cordero-Grande et al.11. The algorithm is compared to a navigated SMS-MUSE3 implementation without intra-volume gross motion correction, called SMS-IRIS. Fractional anisotropy (FA) maps were calculated after affine alignment of the volumes per diffusion direction using Dipy12.
Acquisition
DTI with 4 EPI interleaves, 3 simultaneous slices and 15 diffusion directions at 1000 s/mm2 was performed on a 3T Philips Ingenia scanner with a 32-channel head coil. Image echo parameters were FOV (R x P x S) = 232 x 228 x 120 mm3, resolution = 1.0 x 1.0 x 4.0 mm3, TR = 3 s, TE = 70 ms, FOV/3 CAIPI shift and partial Fourier factor 0.632. The navigator data was acquired at TEnav = 145 ms, 5 mm in-plane resolution, 1.62 in-plane acceleration and FOV/2 CAIPI shift. SPIR was used for fat suppression, and the total scan time was 3:30 min.
The DTI scans were performed twice in five healthy volunteers, first, without motion (static) and, second, with motion. For motion scans, the subjects were asked to remain still in the first third, perform in-plane head shake motion in the second and through-plane nodding motion in the third. Informed consent was attained according to the rules of the institution. Coil reference data was acquired in a gradient-echo prescan.
Results
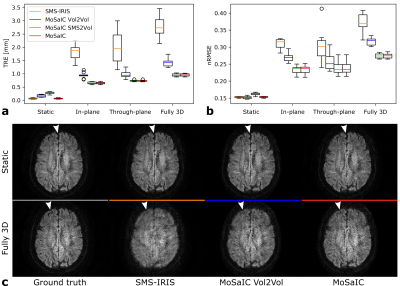
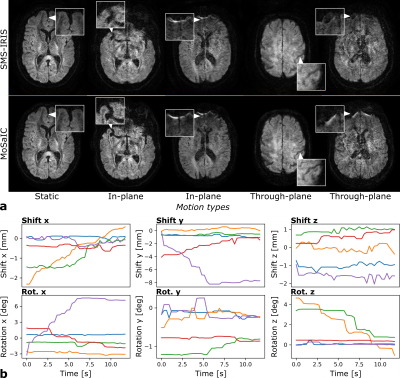
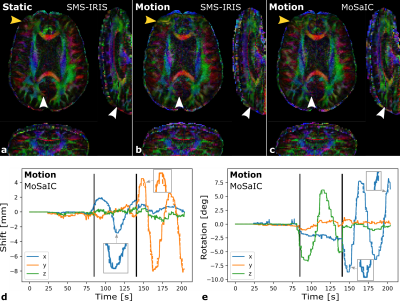
Figure 3 shows the reconstruction and registration performance in different simulated motion scenarios generated from static in-vivo data. The low-resolution navigator analysis enables sub-millimeter target registration errors (TRE)13 and improved image quality for both in- and through-plane motion components. MoSaIC’s motion detection helps reducing errors and computational load in the absence of motion. Motion-corrected in-vivo DWI image volumes and the rigid shot motion parameters are presented in Fig. 4 for different motion cases. Figure 5 compares FA results for in-vivo DTI.Discussion
SMS-to-volume registration, which is itself challenging, is further complicated by the multi-contrast and low-SNR nature of DWI. The presented registration achieves improved image quality over all investigated motion scenarios. Reducing the slice thickness leads to insufficient SNR for the presented method, which currently prevents isotropic DWI and could be addressed in future work by improved slice encoding strategies14.Conclusion
The navigated MoSaIC algorithm integrates the through-plane motion problem to support retrospective full 3D rigid motion correction in interleaved SMS DWI reconstructions, improving image quality in the presence of head motion.Acknowledgements
No acknowledgement found.References
1. Wu W, Miller KL. Image formation in diffusion MRI: A review of recent technical developments: Review of Image Formation in dMRI. JMRI. 2017;46(3):646-662. doi:10.1002/jmri.25664
2. Guhaniyogi S, Chu M-L, Chang H-C, Song AW, Chen N. Motion immune diffusion imaging using augmented MUSE for high-resolution multi-shot EPI: Motion Immune Diffusion Imaging Using AMUSE. MRM. 2016;75(2):639-652. doi:10.1002/mrm.25624
3. Chang H-C, Guhaniyogi S, Chen N. Interleaved diffusion-weighted improved by adaptive partial-Fourier and multiband multiplexed sensitivity-encoding reconstruction: Reconstruction Framework for Artifact-Free DWI. MRM. 2015;73(5):1872-1884. doi:10.1002/mrm.25318
4. Dai E, Ma X, Zhang Z, Yuan C, Guo H. Simultaneous multislice accelerated interleaved EPI DWI using generalized blipped-CAIPI acquisition and 3D K-space reconstruction: SMS Accelerated iEPI DWI. MRM. 2017;77(4):1593-1605. doi:10.1002/mrm.26249
5. Hoinkiss DC, Erhard P, Breutigam N-J, von Samson-Himmelstjerna F, Günther M, Porter DA. Prospective motion correction in functional MRI using simultaneous multislice imaging and multislice-to-volume image registration. NeuroImage. 2019;200:159-173. doi:10.1016/j.neuroimage.2019.06.042
6. Marami B, Scherrer B, Khan S, et al. Motion-robust diffusion compartment imaging using simultaneous multi-slice acquisition. MRM. 2019;81(5):3314-3329. doi:10.1002/mrm.27613
7. Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. MRM. 2013;69(3):793-802. doi:10.1002/mrm.24320
8. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. MRM. 2001;46(4):638–651.
9. Zahneisen B, Ernst T, Poser BA. SENSE and simultaneous multislice imaging: SENSE and Simultaneous Multislice Imaging. MRM. 2015;74(5):1356-1362. doi:10.1002/mrm.25519
10. Beare R, Lowekamp B, Yaniv Z. Image Segmentation, Registration and Characterization in R with SimpleITK. J Stat Soft. 2018;86(8). doi:10.18637/jss.v086.i08
11. Cordero-Grande L, Teixeira RPAG, Hughes EJ, Hutter J, Price AN, Hajnal JV. Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction. IEEE Transactions on Computational Imaging. 2016;2(3):266-280. doi:10.1109/TCI.2016.2557069
12. Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics. 2014;8:8.
13. Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Medical Image Analysis. 2012;16(8):1550-1564. doi:10.1016/j.media.2012.07.004
14. Setsompop K, Fan Q, Stockmann J, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS): High-Resolution Diffusion Imaging With gSlider-SMS. MRM. 2018;79(1):141-151. doi:10.1002/mrm.26653
Figures