1371
Prospective motion-corrected three-dimensional multiparametric mapping of the brain
Shohei Fujita1,2, Naoyuki Takei3, Akifumi Hagiwara1, Issei Fukunaga1, Dan Rettmann4, Suchandrima Banerjee5, Ken-Pin Hwang6, Shiori Amemiya2, Koji Kamagata1, Osamu Abe2, and Shigeki Aoki1
1Department of Radiology, Juntendo University, Tokyo, Japan, 2Department of Radiology, The University of Tokyo, Tokyo, Japan, 3MR Applications and Workflow, GE Healthcare, Tokyo, Japan, 4MR Applications and Workflow, GE Healthcare, Rochester, MN, United States, 5MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 6Department of Radiology, MD Anderson Cancer Center, Houston, TX, United States
1Department of Radiology, Juntendo University, Tokyo, Japan, 2Department of Radiology, The University of Tokyo, Tokyo, Japan, 3MR Applications and Workflow, GE Healthcare, Tokyo, Japan, 4MR Applications and Workflow, GE Healthcare, Rochester, MN, United States, 5MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 6Department of Radiology, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
A rigid real-time prospective motion-corrected multiparametric mapping technique was developed by inserting orthogonal spiral navigators into wait times of a multiparametric imaging technique, 3D-QALAS. The effects of motion correction on the quantitative estimates of a standardized phantom and in volunteers with various head motions were evaluated. Our results demonstrated that the proposed technique did not affect the accuracy of T1 and T2 quantification in vitro, and improved the repeatability and accuracy of T1 and T2 quantification with head motions during scans compared with those without motion correction. The proposed technique may provide robust three-dimensional multiparametric whole-brain mapping for head motions.
Introduction
Multiparametric mapping enables acquisition of multiple tissue properties for non-invasive and objective characterization of biological tissues in a single acquisition.1 A multiparametric technique, 3D-quantification using an interleaved Look-Locker acquisition sequence with T2 preparation pulse (3D-QALAS), combined with parallel imaging and compressed sensing allows high-resolution whole-brain mapping in a clinically feasible time frame.2-4 However, similar to other multiparametric techniques, subject motion during the scan degrades all the mapping, which is one of the major challenges for clinical implementation. One effective approach to compensate subject motion is a real-time prospective motion correction, which was reported to be useful in structural imaging.5,6 Herein, we developed a rigid real-time prospective motion-corrected multiparametric mapping technique by inserting orthogonal spiral navigators into wait times of 3D-QALAS. Subsequently, we evaluated the effects of motion correction on quantitative estimates in a standardized phantom and in volunteers with various head motions.Methods
We integrated a real-time prospective motion tracking and correction technique to the original 3D-QALAS sequence (Fig. 1). Three orthogonal 2D single-shot spiral navigator pulse sequences were inserted into wait times in the original 3D-QALAS acquisition. The reconstructed images of three spiral navigators were input to the extended Kalman filter algorithm7 for tracking and correcting translations and rotations in x-y-z coordinate system. The parameters of the spiral navigator were as follows: FOV, 32 cm; matrix, 128 x 128; thickness, 30 mm; TR/TE, 15.3/0.9 ms; receiver bandwidth, 125 kHz; and, flip angle, 3 °. The flip angle was optimized based on a previous study using a custom phantom.8To evaluate the effects of signal saturation caused by spiral navigators on quantitative mapping, we first evaluated the quantitative accuracy of the proposed technique on the standardized NIST/ISMRM phantom.9 The T1 and T2 values of the clinically relevant range, obtained with spiral navigators, were compared to those without them. The 3D-QALAS imaging parameters were identical, except for the insertion of spiral navigators. The 3D-QALAS sequence imaging parameters were as follows: spatial resolution, 1 × 1 × 1 mm; FOV, 25.6 × 20.5 × 14.6 cm; matrix, 256 × 205 × 146; TR/TE, 8.6/3.5 ms; bandwidth, 25 kHz; flip angle, 5 °; compressed sensing factor, 1.9; parallel imaging factor, 2; and, scan time, 5:56. All subjects were scanned on a 3 T scanner (Discovery MR750w, GE Healthcare, Waukesha, WI) with a 32-channel head coil. The original 3D-QALAS sequence source images were post-processed using SyMRI software prototype ver. 0.45.24 (SyntheticMR, Linkoping, Sweden) to generate T1 and T2 maps.
In vivo images of seven healthy volunteers (6 male and 1 female; mean age ± standard deviation, 30.9 ± 6.2 years) were acquired with the approval of the local institutional review board. Written informed consent was obtained from all study participants. The subjects were scanned once and then instructed to move their heads intentionally during the following scans: in-plane and through-plane movements. For each head motion type, scan-rescan was performed with and without motion correction. T1 and T2 maps were acquired from each head scan. A widely used automated brain segmentation algorithm FIRST, implemented in FSL software version 5.0.9,10 was used to segment brain regions, and these masks were used to obtain regional T1 and T2 values for each scan. These regional values obtained with the proposed approach were compared with those acquired with standard 3D-QALAS without head motion. Scan-rescans for each type of motion were conducted to assess the repeatability of the quantitative values.
Results
Phantom StudyThe T1 and T2 values that were acquired with motion correction showed strong linear correlations (R2 = 0.9990 for T1 and R2 = 0.9992 for T2) with the values acquired with the standard 3D-QALAS without spiral navigators (Fig. 2). The 95% limits of agreement for T1 and T2 ranged from −2.9 to 2.2 ms and −13.3 to 5.7 ms, respectively.
Volunteer Study
Figure 3 shows representative T1 and T2 maps of the brain along with the estimated motion plot obtained from a healthy volunteer with intentional side-to-side (in-plane) motion with and without motion correction. Figure 4 shows representative T1 and T2 maps of the brain along with the estimated motion plot obtained from a healthy volunteer with intentional nodding (through-plane) motion with and without motion correction. The proposed method effectively improved motion robustness. Figure 5 shows bias and repeatability of regional T1 and T2 values of the brain obtained with head motion with and without motion correction. Quantitative values acquired with motion correction showed smaller bias and higher repeatability than those without motion correction.
Discussion
Herein, a motion-robust multiparametric mapping technique was achieved by integrating rigid real-time prospective motion correction into 3D-QALAS. The spiral navigation used in this study did not increase the acquisition time. Our preliminary results indicated that the proposed method could improve the repeatability of T1 and T2 values and reduce bias compared without motion correction. Future studies should evaluate its performance with various real-world movements in patients.Conclusion
The proposed motion correction did not introduce a large bias in the quantitative values obtained in phantoms, and improved accuracy and repeatability of multiparametric maps in volunteer experiments with both in-plane and through-plane motions.Acknowledgements
No acknowledgement found.References
- Seiberlich N, Gulani V, Campbell-Washburn A et al. Quantitative Magnetic Resonance Imaging, Volume 1 – 1st Edition. 2020.
- Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. J Cardiovasc Magn Reson. 2014 Dec 20;16:102. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS.
- Fujita S, Hagiwara A, Hori M, et al. Three-dimensional high-resolution simultaneous quantitative mapping of the whole brain with 3D-QALAS: An accuracy and repeatability study. Magn Reson Imaging. 2019;63:235-243.
- Fujita S, Hagiwara A, Takei N, et al. Accelerated Isotropic Multiparametric Imaging by High Spatial Resolution 3D-QALAS With Compressed Sensing: A Phantom, Volunteer, and Patient Study]. Invest Radiol. 2020;10.1097/RLI.0000000000000744.
- White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, et al. PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking. Magnet Reson Med. 2010;63(1):91–105.
- Watanabe K, Kakeda S, Igata N, et al. Utility of real-time prospective motion correction (PROMO) on 3D T1-weighted imaging in automated brain structure measurements. Sci Rep. 2016;6:38366.
- Kalman RE. A new approach to linear filtering and prediction problems. Trans ASME J Basic Eng 1960;82:35–45.
- Takei N et al. Prospective motion corrected 3D multi-parametric imaging. Proc. Intl. Soc. Mag. Reson. Med. 24 (2020), 0880.
- Keenan et al. Multi-site, multi-vendor comparison of T1 measurement using ISMRM/NIST system phantom. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016), 3290.
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907-922.
Figures
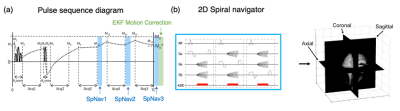
Figure 1. (a) Pulse sequence diagram of the prospective motion-corrected multiparametric mapping. Five data collections were executed at equal time intervals of 1 s. Spiral navigator pulse sequences were inserted into wait times after 3D segmented data acquisition blocks 3, 4, and 5. Extended Kalman filter motion correction was performed after the three spiral navigators. (b) Three orthogonal 2D single-shot spiral navigator pulse sequences for image-based motion tracking. SpNav stands for spiral navigator; Acq stands for acquisition; EKF stands for Extended Kalman filter.
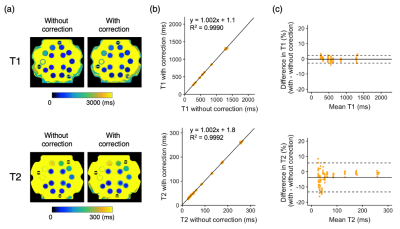
Figure 2. Phantom validation. (a) Quantitative maps of the phantom with and without motion correction. (b) Scatter plots showing the quantitative values obtained with and without motion correction. The linear regression fit is also shown. (c) Bland-Altman plots showing the bias introduced by motion correction. The dotted lines represent the agreement limit, which was defined as the mean difference ±1.96 x SD of the difference between the measurements with and without motion correction.
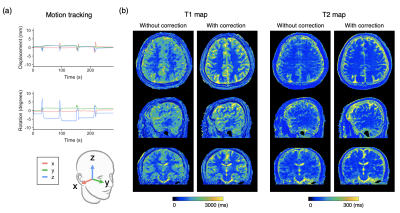
Figure 3. Representative motion tracking and quantitative maps of a healthy volunteer with intentional in-plane (“side-to-side”) head motions. The head motion was rigidly tracked in three translational and three rotational directions. (a) Motion tracking time curve of translations and rotations in the x-y-z coordinate system. (b) T1 and T2 maps acquired with the proposed method and those without motion correction are shown.
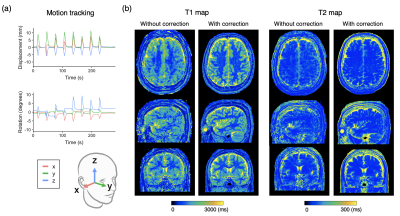
Figure 4. Representative motion tracking and quantitative maps of a healthy volunteer with intentional through-plane (“nodding”) head motions. The head motion was rigidly tracked in three translational and three rotational directions. (a) Motion tracking time curve of translations and rotations in the x-y-z coordinate system. (b) T1 and T2 maps acquired with the proposed method and those without motion correction are shown.
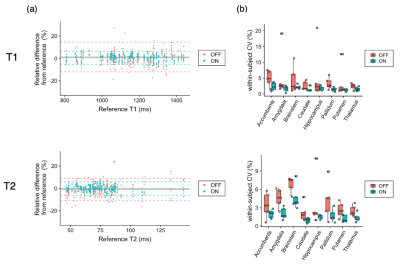
Figure 5. Effect of motion correction on regional quantitative values. (a) Bland-Altman plots representing the bias of scans with motion with and without motion correction compared with scans without motion as references. Data points with motion correction are closer to zero than those without, indicating smaller bias achieved by motion correction. (b) Coefficients of variation (CV) represent the repeatability of the scans. In both T1 and T2, within-subject CVs were smaller, indicating higher repeatability, in motion-corrected scans than those without motion correction.