1356
Deep Learning-Based Respiratory Navigator Echo (DLnav) for Robust Free-Breathing Abdominal MRI1Global MR Applications and Workflow, GE Healthcare Japan, Hino, Japan, 2Department of Radiology, Keio University School of Medicine, Tokyo, Japan
Synopsis
We propose a deep learning-based respiratory navigator (DLnav) technique which uses a convolutional neuronal network (CNN) for respiratory motion detection. The pencil-beam navigator signals were transferred to the real time processing unit including a CNN module and a diaphragm position value was calculated there. DLnav was incorporated into prospectively navigator-gated 3D SPGR and its performance was evaluated in the volunteer scan. DLnav resulted in good synchronization with actual respiratory motion and reduced motion-induced blurring with two different tracker positions.
Introduction
The respiratory navigator echo technique is widely used for free-breathing cardiac and abdominal MRI with minimal respiratory motion artifacts. Navigator echo enables direct respiratory motion detection of the diaphragm and can be used for more accurate respiratory triggering/gating than other techniques with extra devices such as bellows. However, respiratory motion detection with navigator can be degraded due to various causes (e.g. inappropriate navigator tracker positioning), resulting in severe motion artifacts. In this study, we propose a deep learning-based respiratory navigator (DLnav) technique which uses a convolutional neuronal network (CNN) for respiratory motion detection.Methods
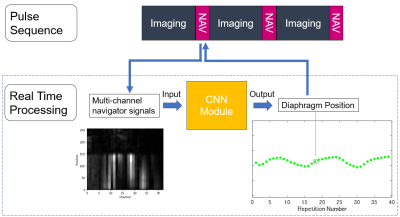
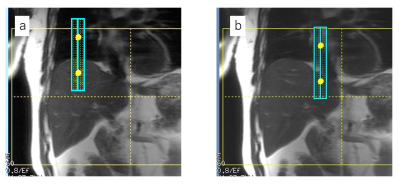
Respiratory Gating with Deep Learning-Based Navigator Echo: The pulse sequence was based on the previously reported navigator-gated 3D SPGR sequence1 (Figure 1a). As shown in Figure 1, a navigator pulse sequence followed an imaging block for acquisition of pencil-beam navigator echo signals. The multi-channel navigator signal data was then transferred to the real time processing unit. The navigator data was used as an input to the CNN module, which includes 4-layer CNN regression model pre-trained with 1115 navigator data sets. The CNN module output a single diaphragm position value (one of the respiratory waveform points in Figure 1), and the value was passed to the pulse sequence and used for imaging data acceptation/rejection in real time (prospective respiratory gating). The navigator processing was performed in 24 ms including the navigator pulse sequence and diaphragm position value calculation with the CNN module.Data Acquisition: We performed experiments on a GE 3 T Pioneer imaging system using floating anterior and embedded posterior coil arrays. The navigator-gated 3D SPGR scans were performed with a healthy volunteer using a 20-mm diameter pencil-beam navigator. 3D data were acquired in the axial orientation with imaging parameters: ARC acceleration factor = 2 × 1, TR/TE = 3.2/1.5 ms, FOV = 40 × 40 cm2, matrix = 256 × 192, slice thickness = 4.4 mm, receiver bandwidth = ±83.3 kHz, flip angle = 10°. The navigator-gated 3D SPGR scan was performed twice, one with the normal tracker positioning (Figure 2a) and the other with the tracker position intentionally shifted onto the heart (Figure 2b). Non-navigator-gated 3D SPGR scan was also conducted for comparison purpose. Approximate scan time was 40 s and 12 s for navigator-gated and non-gated 3D SPGR, respectively.
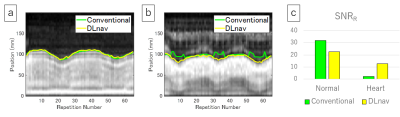
Data Analysis: For respiratory waveform evaluation, a signal-to-noise ratio of respiratory-like frequencies2 (SNRR) was measured. 10-s respiratory waveform data were Fourier transformed in the time domain. The maximum value of the power spectrum within the frequency range for respiration (0.1–0.4 Hz) was divided by the mean value for the frequency range of 0.4–3.0 Hz. This evaluation was also performed for the conventional navigator processing method using the same navigator data sets, and the least-squares error method3 was chosen as the conventional one. For image evaluation, sharpness4 of the left portal vein was quantitatively calculated. Edge values were calculated for both sides of the vessel using a first-order derivative, and final sharpness was defined as the average score of both sides divided by the center of vessel signal intensity.
Results
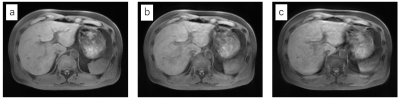
Figure 3 shows the waveforms detected with the conventional and DLnav methods and their SNRR values. The waveforms were well synchronized with the actual respiratory motion and SNRR was higher than 20 for both methods when the tracker was positioned normally. The conventional method’s waveform was degraded (SNRR = 2.2) when the tracker was on the heart due to undesired signals in the superior area, but DLnav maintained a good synchronization with the respiratory motion (SNRR = 12.8). Figure 4 shows 3D-SPGR images. DLnav images reduced motion artifacts and improved the sharpness. Quantitative sharpness values of the portal vein shown in Figure 4 were 0.42, 0.30 and 0.18 for DLnav with normal tracker, DLnav with tracker on the heart and non-gating, respectively.Discussion
We have developed the DLnav technique and have shown that navigator-gated 3D SPGR with DLnav is feasible. The respiratory waveform had a high SNRR even when the tracker was positioned badly on the heart. Though DLnav images had higher sharpness values for two different tracker potions than the non-gated image, comparison between different tracker positions shows that the tracker on the heart resulted in blurring even with DLnav when compared to the normal tracker position. However, the motion detection and resultant 3D SPGR image quality can be improved further with increased number of training navigator signal data sets of various scan conditions. Clinical evaluation with a large number of subjects is required in future.Conclusion
The DLnav technique is feasible and improved robustness of free-breathing abdominal MRI in the volunteer scan.Acknowledgements
No acknowledgement found.References
1. Vasanawala SS, Iwadate Y, Church DG, et al. Navigated abdominal T1-W MRI permits free-breathing image acquisition with less motion artifact. Pediatr Radiol. 2010;40:340–4.
2. Walker MD, Morgan AJ, Bradley KM, et al. Evaluation of data-driven respiratory gating waveforms for clinical PET imaging. EJNMMI Res. 2019;9:1.
3. Wang Y, Grimm RC, Felmlee JP, et al. Algorithms for extracting motion information from navigator echoes. Magn Reson Med 1996;36:117–123.
4. Botnar RM, Stuber M, Danias PG et al. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99:3139–3148.
Figures