1340
Quantitative Accuracy of Diffusion-Weighted Imaging Techniques as a Function of Susceptibility Artifact Resilience1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Center for Imaging Research, Medical College of Wisconsin, Milwaukee, WI, United States, 3Canon Medical Research USA, Cleveland, OH, United States
Synopsis
Diffusion weighted imaging(DWI) is a workhorse sequence for many clinical and research applications of MRI. Beyond the role in neuroimaging, DWI has also been deployed for use other anatomies outside the head. However, in some anatomies with air-tissue susceptibility boundaries or proximity to metallic implants, DWI assessment may be challenging due to substantial susceptibility artifacts. In this study, the diffusion quantification accuracy of several DWI sequences within increasing resilience to susceptibility artifacts were compared using an established DWI phantom. In addition, a controlled test of two sequences with substantial differences in susceptibility artifact reduction was performed on spinal cord DWI.
Introduction
While DWI can provide valuable qualitative tissue contrast for clinical diagnostics, it has substantial untapped potential strengths as a quantitative clinical imaging biomarker. This potential quantification strength of DWI is counter-balanced by its vulnerability to susceptibility artifacts. Susceptibility artifacts conventionally found in DWI, which are based upon its traditional reliance on single-shot echo-planar imaging (SS-EPI) methods with low phase-encoding direction pixel bandwidths. There are several modifications to EPI that can reduce susceptibility artifacts, including acquisition segmentation1,2,3 and the use of parallel imaging.Refocused spin-echo DWI techniques can also be applied by either compensating for Carr-Purcell-Meiboom-Gill (CPMG) violations4,5 or by utilizing a DWI preparation module before the data acquisition train6. Additionally, a new approach to DWI in the presence of metallic hardware leverages both CPMG mitigation and multi-spectral imaging techniques7.
In this study 4 different methods are analyzed for quantitative accuracy and consistency: commercially available single-shot EPI and MUSE3 (segmented EPI), DW-PROPELLER (DW-Prop)4 and a DW MSI-PROPELLER (MSI-Prop) metal-artifact reduction prototype. Collectively, these sequences have theoretical susceptibility artifact resilience capabilities that scale by over an order of magnitude, ranging from single-shot EPI to MSI-PROPELLER.
Methods
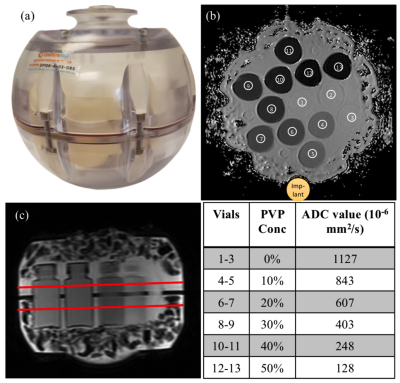
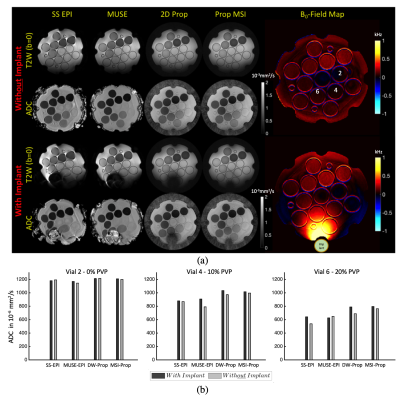
Experiments were performed on a GE Premier 3T MRI scanner. Phantom experiments were performed with NIST diffusion phantom shown in Fig.1. The NIST phantom contains vials filled with polymer solution concentration at 0 to 50% with the measured ADC referenced using the vendor-recommended gold standard protocol as given in Fig.1.To test the different techniques the following acquisition parameters were set to: FOV=22cm, matrix size=128x128, 4mm 5 slices with slice gap=1mm, ETL=20, BW=62.5kHz, TR=4s and 3 diffusion directions. 3 spectral bins were acquired for the MSI-Prop technique. Three acquisitions were done corresponding to one of three b-values =350s/mm2, 600s/mm2, 1000s/mm2. The experiments were repeated 3 times. Another phantom experiment collected similar data with and without a large cobalt-chromium implant next to the phantom, as shown in Fig.3(a).
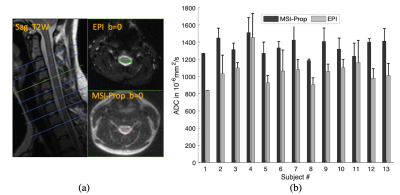
ADC maps were calculated using a mono-exponential fit. The mean ADC was calculated in 6-pixel diameter circular regions of interest (ROI) in the vials as shown in Fig.1(b). The values within the ROIs were used within k-fold (k=10) cross validation approach to account for multiple voxel measurements per vial. ADC biases and trends were analyzed using generalized linear fit model in Matlab. Written informed consent was obtained from the 13 volunteers prior to their participation in a study for an IRB-approved study. All volunteers were healthy controls with asymptomatic spines. Because these subjects were enrolled in a study with additional acquisitions for alternative aims, only 2 techniques, MSI-Prop and single-shot EPI were acquired with b=600s/mm2 on the cervical spine as shown in Fig.4. After pre-processing the data using the Spinal Cord Toolbox8, average ADC values were calculated for every cervical spine level in which data was acquired.
Results
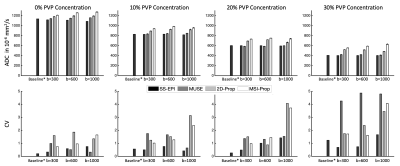
Fig.2 provides ADC values for each of the 4 vial groups (ADC (403, 1127)·10-6mm2/s). ADC accuracy improved with increasing b-value, though the differences in ADC values stayed relatively consistent across vials. The DW-Prop and MSI-Prop consistently overestimated the ADC values especially for lower b-values. Regression analysis showed that the biases were smaller than 29·10-6mm2/s for EPI based acquisitions. The bias range was (83, 151)·10-6mm2/s for for DW-Prop and (192, 232)·10-6mm2/s for MSI-Prop. All of the computed linear coefficients were in (-0.10, 0.01) range. The coefficient of variation of the sequences (based on multiple experiment runs) increased with b-value and reduced with vial ADC. Maximum ranges of CV were found at the 4-5% level in MUSE-EPI and PROPELLER-based methods in the lower ADC vials when using higher b-values.Fig. 3 provides qualitative assessment of susceptibility artifact resilience and the result of the susceptibility variations on DW quantification.
Spinal cord volunteer results are provided Fig.4. In this volunteer cohort, MSI-Prop showed a positive bias relative to FOCUS EPI with an amplitude of roughly (20%) 293·10-6mm2/s These results are roughly aligned with the phantom experiment results (bias: 209·10-6mm2/s for b=600s/mm2), though there are substantial.
Discussion
While the EPI techniques can provide an efficient and often logistically superior approach to diffusion-weighted imaging, they lack the ability to image near metal or anatomies that induce severe susceptibility artifacts. MUSE-EPI can be deployed to manage more mundane susceptibility issues, and shows good quantitative agreement with SS-EPI approaches. However, the results of this analysis indicate that MUSE does come with a cost of increased test-retest variation for lower ADCs and higher b-values.PROPELLER-based DW acquisitions are far less prone to susceptibility artifacts and allow for imaging in extreme susceptibility environments. However, these advantages come at a cost of bias increase of in ADC values and similar test-retest increases as observed with MUSE EPI.
The source of the PROPELLER-based ADC positive bias remains under investigation but is hypothesized to be caused by the unique non-Cartesian PROPELLER acquisition and possibly its interplay with non-CPMG mitigation techniques used in the PROPELLER DW acquisition approach. Further work will continue exploring the mitigation of this effect and/or a calibration process to allow for robust comparisons of EPI and PROPELLER-based DW approaches.
Acknowledgements
Research reported in this publication was partially supported by grants from the Department of Defense (W81XWH1910273), NIH (NIH R21EB023415), and the Daniel M. Soref Charitable Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, NIH, or the Daniel M. Soref Charitable Trust.References
[1] Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout-segmented EPI for rapid high resolution diffusion imaging at 3T. European Journal of Radiology 2008;651:36–46.
[2] Skare S, Newbould RD, Clayton DB, Bammer R. Propeller EPI in the other direction. Magnetic Resonance in Medicine 2006;556:1298–1307.
[3] Chen Nk, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41–47.
[4] Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2002;471:42–52.
[5] Schick, Fritz. "SPLICE: sub‐second diffusion‐sensitive MR imaging using a modified fast spin‐echo acquisition mode." Magnetic resonance in medicine 38, no. 4 (1997): 638-644.
[6] Yamashita, Koji, Takashi Yoshiura, Akio Hiwatashi, Makoto Obara, Osamu Togao, Nozomu Matsumoto, Kazufumi Kikuchi, and Hiroshi Honda. "High-resolution three-dimensional diffusion-weighted imaging of middle ear cholesteatoma at 3.0 T MRI: Usefulness of 3D turbo field-echo with diffusion-sensitized driven-equilibrium preparation (TFE–DSDE) compared to single-shot echo-planar imaging." European Journal of Radiology 82, no. 9 (2013): e471-e475.
[7] Koch KM, Bhave S, Gaddipati A, Hargreaves BA, Gui D, Peters R, Bedi M, Mannem R,Kaushik SS. Multispectral diffusion-weighted imaging near metal implants. Magnetic resonancein medicine 2018;792:987–993.
[8] De Leener B, Levy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, Callot V, Cohen-Adad J. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2016
Figures