1338
Flip-angle optimization for the diffusion-weighted SPLICE sequence for applications in brain imaging1Department of Health Technology, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 3Laboratory of Radiation Physics, Odense University Hospital, Odense, Denmark, 4Department of Clinical Research, University of Southern Denmark, Odense, Denmark, 5Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 6Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kgs. Lyngby, Denmark
Synopsis
The RARE-based diffusion-weighted MRI sequence, SPLICE, provides diffusion-weighted images free of geometric distortions. However, the image quality in terms of spatial resolution and SNR needs to be improved. We suggest a framework based on optimization of the flip-angles in the refocusing pulse train to improve the SNR for a chosen spatial point-spread-function. Simulations based on EPG calculations indicate superiority of the optimized flip-angle scheme and experimental recordings on a 1.5 T scanner demonstrate the improvements in a practical setting.
Introduction
Diffusion weighted imaging reflects the microanatomy of tissue, as it probes water molecule displacement. As tumors consist of dense tissue, the motion restriction results in a high signal intensity in tumors compared to surrounding tissues. Therefore, the clear contrast of diffusion-weighted MRI can help the tumor delineation process. Currently, echo-planar imaging (EPI) readout is standard for DW-MRI scans. However, geometrical distortions from static field inhomogeneity are problematic for radiotherapy applications, for example. An alternative DW-MRI sequence is SPLICE, which employs a rapid acquisition with relaxation enhancement (RARE) readout module with separation of families of echoes with different phase1. The method is robust to geometrical distortions2, but affected by T2-weighted signal modulation during readout resulting in blurred images. This work presents a framework for optimization of the SPLICE sequence, where the refocusing flip-angles (FAs) are designed specifically to improve the spatial point-spread-function (PSF) and SNR to reduce blurriness and increase image quality. Earlier work on FA optimization have focused on standard RARE, and typically on reducing SAR3,4. We present simulations used for optimization as well as early experimental validation on a 1.5 T scanner.Material and methods
The FAs were optimized for a predefined target function, $$$T(k)$$$, describing the k-space density weighting throughout the echo train. This corresponds to a desired PSF (related through the Fourier transform) that is essentially the voxel shape in the phase-encoding direction. The signal modulation during the echo train, the modulation transfer function, $$$MTF(k)$$$, depends on the FAs and was obtained using an extended phase graph (EPG) simulation5. A characteristic feature of the SPLICE sequence is a prolonged and imbalanced read-out gradient to split echoes with different phases into two families, such that artifacts from violated CPMG conditions are avoided. The signal for both echo families were exemplarily simulated for a set of 63 refocusing pulses (ETL) with a given FA scheme, using an echo spacing (ESP) of 5.2 ms, and approximate average brain tissue relaxation parameters (T1=1000 ms, T2=100 ms). A filter, $$$F(k)$$$, is chosen to ensure that the resulting images has the target PSF, i.e.$$F(k)=\frac{T(k)}{MTF(k)} $$
The optimal FAs were obtained by maximizing the SNR depending on the compensating filter that should preferably fulfill $$$F(k)\approx1$$$ meaning that the flip-angle scheme implements acquisition weighting for the chosen PSF with little need for filtering:
$$\text{SNR}\propto\frac{1}{∑_{i=1}^{\text{ETL}}F(k_i )}$$
An exemplary Hanning weighting function was chosen as target6 (Figure 1a) resulting in the PSF seen in Figure 1b. The FAs were optimized using a nonlinear programming solver utilizing an interior-point method for both linear and center-out order of phase-encoding steps in k-space (simulation results are only presented for the linear ordering). Simulated SNR for an optimized FA scheme was compared to a reference scheme consisting of repeated, mostly constant 120° flip-angle (Figure 1c), representing a standard clinical RARE sequence.
The data
Brain scans were collected for a healthy subject on a 1.5 T MRI Philips Ingenia system using the SPLICE sequence (ETL=63, ESP=5.2 ms, b-values=0; 800 s/mm2 (three orthogonal directions)) with the optimized FA scheme and the reference scheme, respectively. Raw coil-array k-space data was reconstructed and combined using coil sensitivities7 estimated from b=0 data after applying the calculated filters. Magnitude images from each echo family were added to form a final image. Simulation and processing steps were carried out using Matlab2018b (The Mathworks, Natick, MA).
Results and Discussion
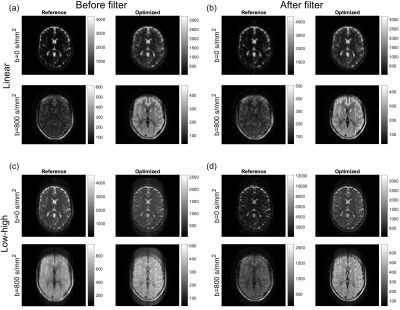
The optimal FA scheme (linear sampling) is very different from the constant reference scheme (Figure 1c) but is similar to pseudo-steady-state optimized schemes presented in the literature3,8. The oscillating behavior of the resulting filters (Figure 1d) is expected as they compensate for small oscillations in each echo family, an effect of alternating spin echoes and stimulated echoes between families. The relative SNR improvement for the proposed scheme compared to the reference was 1.70 for linear sampling and 1.71 for center-out sampling.The images of the brain (Figure 2) show a visual improvement using the proposed FA schemes, especially for b=800 s/mm2, which indicate a general improvement in SNR. The optimized data showed corresponding substantial improvements in SNR of estimated ADC maps (not shown). Especially for linear k-space filling, the effective resolution is increased and ringing artifacts are reduced, consistent with the chosen PSF. This improvement is less obvious for the center-out k-space filling, where ghosting artifacts affect the images. This is due to initial oscillations in the echo families occurring during sampling of the central k-space, where the filters become important (comparing Figure 2c with 2d). The unfiltered data is included to demonstrate the effect of the filters, which are tissue-specific (optimized for T1=1000 ms, T2=100 ms), and thus may not effectively improve image quality in all tissue types. The filtering has almost no impact on the linear data, where the optimized FA scheme alone improves the images.
Conclusion
Optimization of the set of flip-angles used in the echo train of a SPLICE sequence can improve the image quality for the desired spatial resolution and voxel shape as expressed by the PSF. We showed a reduction in effective voxel size (decreased blur) and an increase in SNR. The method offers flexible implementation of DW-MRI sequences without geometrical distortions, e.g. for radiotherapy planning.Acknowledgements
Sofie Rahbek was funded by the Danish Cancer Society (grant R167-A10637-17-S2).References
1. Schick F. SPLICE: Sub-second diffusion-sensitive MR imaging using a modified fast spin-echo acquisition mode. Magn Reson Med. 1997;38(4):638-644. doi:10.1002/mrm.19103804182.
2. Schakel T, Peltenburg B, Dankbaar JW, et al. Evaluation of diffusion weighted imaging for tumor delineation in head-and-neck radiotherapy by comparison with automatically segmented 18F-fluorodeoxyglucose positron emission tomography. Phys Imaging Radiat Oncol. 2018;5(December 2017):13-18. doi:10.1016/j.phro.2017.12.0043.
3. Busse RF, Brau ACS, Vu A, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med. 2008;60(3):640-649. doi:10.1002/mrm.216804.
4. Loening AM, Saranathan M, Ruangwattanapaisarn N, Litwiller D V., Shimakawa A, Vasanawala SS. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J Magn Reson Imaging. 2015;42(6):1747-1758. doi:10.1002/jmri.249415.
5. Hennig J, Weigel M, Scheffler K. Calculation of Flip Angles for Echo Trains with Predefined Amplitudes with the Extended Phase Graph (EPG)-Algorithm: Principles and Applications to Hyperecho and TRAPS Sequences. Magn Reson Med. 2004;51(1):68-80. doi:10.1002/mrm.106586.
6. Pohmann R, Von Kienlin M. Accurate phosphorus metabolite images of the human heart by 3D acquisition-weighted CSI. Magn Reson Med. 2001;45(5):817-826. doi:10.1002/mrm.11107.
7. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR Phase array. Magn Reson Med. 1990;16(2):192-225.8.
8. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: Design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55(5):1030-1037. doi:10.1002/mrm.20863
Figures

The Hanning target function (a) and corresponding PSF (b) having a low FWHM and limited ripples. The optimized FAs (c) together with the reference scheme of 120° flips. The first four echoes are marked as “transients” and discarded for improved robustness. The resulting filters for both echo families, E1 and E2, normalized with the coefficient used for the center of k-space.