1322
Navigator-Free Submillimeter Diffusion MRI using Multishot-encoded Simultaneous Multi-slice (MUSIUM) Imaging
Wei-Tang chapel Chang1, Khoi Minh Huynh2, Pew-Thian Yap1, and Weili Lin2
1Radiology, UNC at Chapel Hill, Chapel Hill, NC, United States, 2BRIC, UNC at Chapel Hill, Chapel Hill, NC, United States
1Radiology, UNC at Chapel Hill, Chapel Hill, NC, United States, 2BRIC, UNC at Chapel Hill, Chapel Hill, NC, United States
Synopsis
One major challenge in submillimeter dMRI is the inherently low signal-to-noise ratio (SNR). to To address this issue, the simultaneous multislab (SMSlab) approaches were proposed but susceptible to slab boundary artifacts and require additional navigators for phase estimation. The gSlider sequences require relatively high RF power and peak amplitude, increasing SAR and complicating RF excitation. Here, we introduce a navigator-free approach called multishot-encoded simultaneous multi-slice (MUSIUM) imaging for enhanced SNR, low RF power and peak amplitude, and freedom from slab boundary artifacts.
Introduction
Major impediments in submillimeter dMRI include long acquisition time and inherently low signal-to-noise ratio (SNR). While the simultaneous multislice (SMS) approach is capable of shortening acquisition time considerably, the low SNR remains an issue. Two different approaches were proposed to increase both excitation volume per shot and number of shots, namely simultaneous multislab (SMSlab)1-3 dMRI and Generalized Slice Dithered Enhanced Resolution with Simultaneous Multislice (gSlider-SMS)4. The SMSlab approach enables rapid acquisition and enhances SNR but suffers from slab boundary artifacts, insufficient SNR per shot for phase estimation, and potential Gibbs artifacts. gSlider is an RF-encoded multishot approach that achieves submillimeter resolution at the cost of elevated SAR and RF peak amplitude. Here, we introduce multishot-encoded simultaneous multi-slice (MUSIUM) imaging, an approach for enhanced SNR, low RF power and peak amplitude, motion robustness, and freedom from slab boundary artifacts. Each encoding shot of MUSIUM imaging has sufficient SNR for phase self-navigation. Whole-brain dMRI with 0.86-mm isotropic resolution and 66 diffusion directions can be acquired in ~12.5 minutes.Method
As shown in Figure 1a, nsms×nshot slices are excited simultaneously for each shot. The fully sampled k-space of SMS can be viewed as the Fourier encoding of a 3D volume comprising nsms×nshot contiguous slabs that are centered around the excited slices (Fig 1a, dashed lines)5. The fully-sampled k-space points in Figure 1a are denoted as orange circles in Figure 1b. In contrast to conventional 3D encoding, SMS 3D encoding is virtually free from Gibbs ringing effect6 regardless of the number of partition encoding (PAE). This is because the image signal along the slice direction is discrete instead of continuous. Therefore, the Fourier transform of the image signal can be approximated as discrete Fourier transform in which Gibbs phenomenon is not applicable.All experiments were performed using a 3T Siemens Prisma scanner. Imaging studies were conducted to 1) compare the SNR between conventional SMS and MUSIUM sequences, and 2) demonstrate the efficacy of MUSIUM in submillimeter whole-brain dMRI. The imaging protocols are as follows: matrix dimension=256×208×153 (R-L×H-F×A-P) mm3; 0.86 mm isotropic resolution; axial slicing; SMS factor = 3; number of shots = 3, TE = 99 ms; TR per shot = 3.65 s and effective TR = 10.95 s. There were 5, 15, 45 diffusion directions at b=500, 1000, 2000 s/mm2 respectively with 1 b0 image. Image reconstruction involves phase estimation and SENSE-based reconstruction.Results
The SNR comparison between single-shot SMS and multi-shot MUSIUM acquisitions is demonstrated in Figure 2. The number of shots in MUSIUM sequences is 3. Figure 2a shows the SNR maps of SMS and MUSIUM and Figure 2b shows the corresponding ratio maps. In general, SNR is enhanced by MUSIUM imaging with an average SNR increase of 56.2% over SMS imaging.Representative diffusion-weighted images with 0.86 mm isotropic resolution are shown for different b-values in Figure 3a. The color-FA maps are shown in Figure 3b with the tensor principal directions shown in the zoomed-in view at the bottom right. The principal directions in the cortex are generally perpendicular to the white-gray matter interface. A total of 66 images were acquired with ~12.5 minutes.Figure 4 shows the effects of the motion for three different conditions. Figure 4a shows the images without motion as refence. The linear translation of 1 mm and linear rotation of 1 degree during MUSIUM encoding lead to slight stripping artifacts as shown in Figure 4b and the artifacts become more pronounced in Figure 4c. The general features of images are preserved despite the presence of motions.Discussion
We have proposed a novel approach for ultra-high isotropic resolution, whole-brain dMRI within a clinically acceptable acquisition time. We demonstrated several major advantages of the proposed MUSIUM imaging approach over existing state-of-the-art approaches. MUSIUM simultaneously excites a large number of slices and employ multi-shot to enhanceSNR and reduce g-factor penalty. The k-space sampling trajectories were designed in a way that the SNR of each shot is sufficient for phase estimation. Compared with gSlider, MUSIUM imaging can be implemented on MR scanners without increasing the RF pulse duration, dedicated high performance gradient coils and the use of complicated RF excitation schemes such as VERSE. In vivo MUSIUM images show higher SNR efficiency than SMS images. The efficacy of submillimeter MUSIUM imaging was demonstrated at 0.86 mm isotropic resolution, revealing detailed structures in cortical and white matter areas.Acknowledgements
We thank Kim-Han Tung and Ye Wu for providing helpful comments. This work was supported in part by NIH grants, R21AG060324 and U01MH110274.References
1. Frost, R., et al. Simultaneous multi-slab acquisition in 3D multi-slab diffusion-weighted readout-segmented echo-planar imaging. in ISMRM 21th Annual Meeting & Exhibition. 2013. Salt Lake City, Utah, United States.2. Chen, L. and D.A. Feinberg. Simultaneous Multi-Slab Echo Volume Imaging: comparison in sub-second fMRI. in ISMRM 21th Annual Meeting & Exhibition. 2013. Salt Lake City, Utah, United States.3. Bruce, I.P., et al., 3D-MB-MUSE: A robust 3D multi-slab, multi-band and multi-shot reconstruction approach for ultrahigh resolution diffusion MRI. Neuroimage, 2017. 159: p. 46-56.4. Setsompop, K., et al., High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn Reson Med, 2017.5. Zahneisen, B., et al., Three-dimensional Fourier encoding of simultaneously excited slices: Generalized acquisition and reconstruction framework. Magn Reson Med, 2013.6. Czervionke, L.F., et al., Characteristic features of MR truncation artifacts. AJR Am J Roentgenol, 1988. 151(6): p. 1219-28.Figures
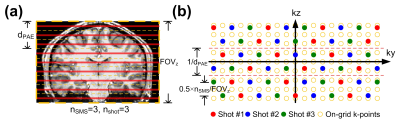
Figure 1: The RF excitation and k-space sampling trajectories of the MUSIUM sequences. (a) The RF excitation of a MUSIUM sequence. nsms × nshot slices are excited simultaneously. (b) The sampling trajectories of MUSIUM sequence.
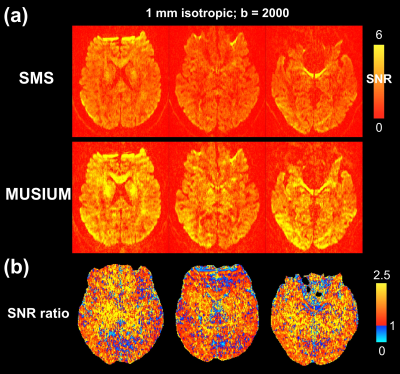
Figure 2: The SNR comparison between single-shot SMS and multi-shot MUSIUM acquisitions. (a) The SNR maps of SMS and MUSIUM acquisitions. (b) The SNR ratio of MUSIUM to SMS. The SNR values and ratios are color-encoded as indicated by the color bars.
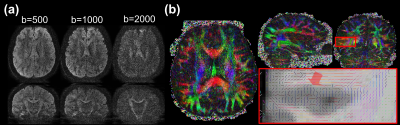
Figure 3: The reconstruction results of the 0.86-mm isotropic MUSIUM data. (a) The diffusion images at b=500, 1000 and 2000. (b) The FA map in three orthogonal planes. The data acquisition time is ~12.5 minutes.
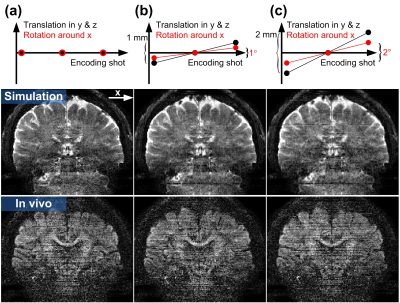
Figure 4: The motion effects on the MUSIUM reconstruction. Three motion conditions along the 3 encoding shots are simulated: (a) No motion, (b) linear translation of 1 mm in y and z axes and linear rotation of 1° around x axis, and (c) linear translation of 2 mm in y and z axes and linear rotation of 2° around x axis. The simulated and in vivo images are shown in the middle and bottom rows of each panel respectively.