1320
Highly Segmented Multishot Diffusion Imaging With Spiral Readouts1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zürich, Switzerland
Synopsis
Multi-shot acquisition for diffusion MRI is challenging due to shot-to-shot phase variations caused by motion. Multiplexed sensitivity-encoding (MUSE) tackles this problem by extracting phase estimates from reconstruction of individual shot images. In this approach, the feasible number of shots is limited by increasing g-factor noise penalty. Against this background, the present work studies the feasibility of highly segmented MUSE with spiral acquisition, which offers particularly benign g-factor behavior. To stabilize reconstruction, we explore the utility of moving from separate estimation of phase offsets to joint optimization of phase biases and image content.
INTRODUCTION
Diffusion MRI data have been mostly acquired with single-shot echo-planar-imaging (EPI) because single-shot techniques are fast and robust against motion during diffusion encoding, particularly against pulsatile brain motion1. However, this approach is limited to the spatial resolution that can be achieved with just a single readout. Higher resolution is afforded by multiple-shot, segmented acquisitions. These, however, require special reconstruction schemes that correct for shot-to-shot phase variations due to motion during diffusion encoding. Multiplexed sensitivity-encoding (MUSE)2 relies on separate parallel imaging reconstruction of individual segments to estimate phase maps and subsequently obtain a joint image from all segments. The phase estimation is challenging when readouts are highly segmented and thus highly undersampled individually, causing strong g-factor penalty and increased artifact due to ill-conditioning. Against this background, an interesting option for highly segmented diffusion imaging is spiral acquisition, which has recently been found to offer more benign g-factor behavior than EPI3.On this basis, the present work studies the feasibility of highly segmented diffusion imaging with spiral acquisition and MUSE reconstruction. To stabilize reconstruction, we additionally explore the utility of joint optimization of phase biases and image content.
METHODS
Theory: Reconstruction of multi-shot diffusion data can be formulated as the minimization of$$L = ||m-E\Phi O||_2^2 + \lambda||\nabla^2 \phi_{vc}||_2^2. \qquad \textrm{[1]}$$
by varying the object vector O [N2×1] and the vector $$$\phi_{vp}$$$ [nsN2×1] of phase biases for all shots, given the measured signal m [nsnknc×1], the block diagonal encoding matrix E [nsnknc×nsN2], which incorporates coil sensitivities, the k-space trajectory, and B0 inhomogeneity. $$$\Phi$$$ of size [nsN2×N2] is a stack of diagonal matrices with entries $$$e^{i\phi_{vp,(j,x)}}$$$, $$$\phi_{vc}$$$ of size [nsN2×1] lists the same phasors in a vector, and λ is a regularization parameter. ns, nk, nc, and N2 represent the number of shots, sampling points, coil elements and image voxels, respectively. In Eq. [1], the first term ensures data fidelity and the second penalizes roughness. The derivatives of the loss function with respect to O and $$$\phi_{vp}$$$ are
$$\frac{\partial L}{\partial O}=-2\Phi^HE^H (m-E\Phi O)\qquad \textrm{[2.1]}$$
$$\frac{\partial L}{\partial \phi_{vp}}=2Re\left[i \cdot{} diag\left(\left(\Phi O\right)^H\right)E^H\left(m-E\Phi O\right)\right]+2Re\left[-i \cdot{} diag\left(\phi_{vc}^H\right)\left(\nabla^2\right)^H)\nabla^2\phi_{vc}\right]. \qquad \textrm{[2.2]}$$
Data Acquisition: One healthy volunteer was scanned under local ethics approval with a 3T Philips Achieva scanner and gradients operated in parallel mode (Gmax=80mT/m, slew-ratemax=100mT/m/ms). A 16ch receive-only brain array with 16 integrated 19F NMR probes (NeuroCam, Skope MR Technologies, Zurich, Switzerland) was employed to collect MRI raw data and concurrently monitor magnetic field evolution. For mapping B0 and coil sensitivities, multi-echo GRE images (TE/ΔTE=2.3/1.15ms) were acquired. Four spiral diffusion datasets (b=1000s/mm2) were acquired with varying number of shots (ns=4,8,12,16) and readout durations (TRO=54,27,18,14ms) at the resolution of 0.8×0.8×2.0mm3.
Image Reconstruction: Diffusion datasets were reconstructed using the MUSE method. To stabilize reconstruction upon strong segmentation, the loss (Eq. [1]) was minimized by strict gradient descent, using the analytical gradients (Eq. [2]). As Eq. [1] is non-convex, appropriate initialization is important. The MUSE result was used to initialize O and $$$\phi_{vp}$$$. In each iteration, a line search was performed along the gradient direction, updating O and $$$\phi_{vp}$$$ simultaneously.
From concurrently monitored phase evolutions of NMR probes, 3rd-order phase expansions in terms of spherical harmonics were determined, including 2nd-order concomitant field correction4. These phase expansions, B0 and sensitivity maps were used to set up the encoding matrix in all reconstructions5.
RESULTS
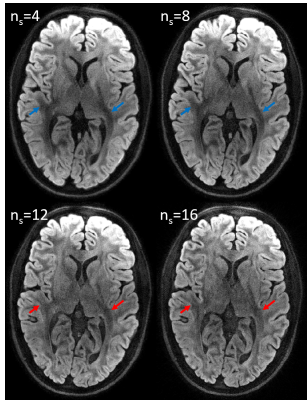
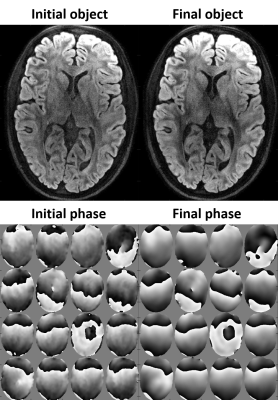
Fig. 1 shows multi-shot diffusion-weighted images (DWIs) reconstructed using the MUSE algorithm. Even at high segmentation, high image quality was obtained. This is remarkable considering that reconstruction from individual shots is vastly ill-conditioned in these cases. Nevertheless, the DWIs with 12- and 16-fold segmentation exhibit less and partly altered diffusion contrast (blue vs. red arrows). As shown in Fig. 2, the proper contrast strength and structure were recovered by joint optimization of phase biases and image content.DISCUSSION
Multi-shot DWIs with highly segmented spiral readouts were successfully reconstructed with high image quality, resolution and high SNR even without averaging and not requiring navigator data. The MUSE method provided high image quality even with strong segmentation. This is attributed to the relatively benign scale and spatial structure of the g-factor for spiral readouts as well as to the accuracy of the signal model (readout trajectories, B0 and sensitivity maps). Nonetheless, slight alteration of diffusion contrast was observed in the highly segmented images. This effect was overcome by weighted minimization of signal model violation and roughness of phase biases. Unlike MUSE, joint estimation of image content and phase bias is not prone to error propagation from ill-conditioned phase estimates based on single shots.CONCLUSION
Spiral readouts readily permit highly segmented diffusion imaging without navigation. MUSE processing yielded robust image reconstruction up to intermediate segment counts. Joint optimization of phase biases and image content expanded the feasible range up to 16 segments. Expanded feasibility of segmented diffusion scanning is a promising prospect in that it reconciles high resolution with intrinsic limits on readout duration while maintaining the time efficiency of standard scanning without navigation or segmentation in the frequency encoding direction.Acknowledgements
Technical support from Philips is gratefully acknowledged.References
1. Miller KL and Pauly JM. Nonlinear Phase Correction for Navigated Diffusion Imaging. Magn Reson Med 2003;50:343-353.
2. Chen N‐K, Guidon A, Chang HC, et al. A robust multi‐shot scan strategy for high‐resolution diffusion weighted MRI enabled by multiplexed sensitivity‐encoding (MUSE). NeuroImage 2013;72:41–47.
3. Lee Y, Wilm BJ, Brunner DO, et al. On the signal-to-noise ratio benefit of spiral acquisition in diffusion MRI. Magn Reson Med 2020 early view.
4. Wilm BJ, Nagy Z, Barmet C, et al. Diffusion MRI with concurrent magnetic field monitoring. Magn Reson Med 2015;74:925-933.
5. Wilm BJ, Barmet C, Gross S, et al. Single-Shot Spiral Imaging Enabled by an Expanded Encoding Model: Demonstration in Diffusion MRI. Magn Reson Med 2017;77:83-91.
Figures